正在加载图片...
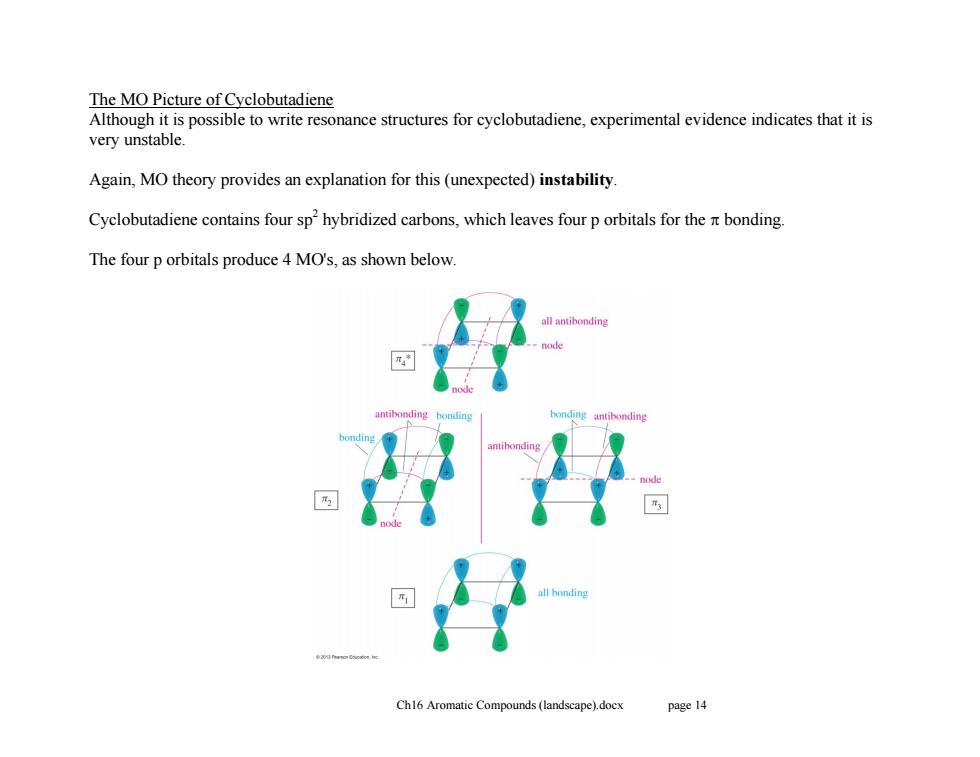
The MO Picture of Cyclobutadiene Although it is possible to write resonance structures for cyclobutadiene,experimental evidence indicates that it is very unstable. Again,MO theory provides an explanation for this (unexpected)instability Cyclobutadiene contains four sphybridized carbons,which leaves four p orbitals for the bonding. The four p orbitals produce 4 MO's,as shown below. Ch16 Aromatic Compounds (landscape).docx page 14Ch16 Aromatic Compounds (landscape).docx page 14 The MO Picture of Cyclobutadiene Although it is possible to write resonance structures for cyclobutadiene, experimental evidence indicates that it is very unstable. Again, MO theory provides an explanation for this (unexpected) instability. Cyclobutadiene contains four sp2 hybridized carbons, which leaves four p orbitals for the bonding. The four p orbitals produce 4 MO's, as shown below