正在加载图片...
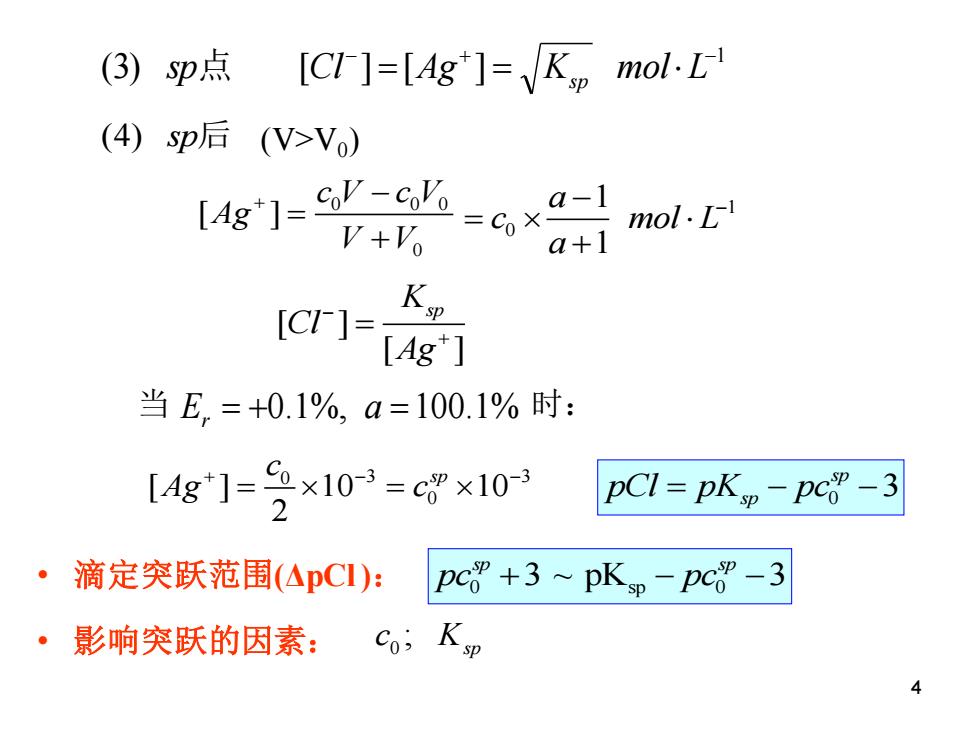
(3)p点 [CI-]=[Ag"]=K mol.L (4)sp后(V>Vo) [g]=c业=6×a V+Vo mol.L a+1 [C]= [Ag*] 当E,=+0.1%,a=100.1%时: L4g]-=2×10=cgx10 pCl=pKp-pc”-3 滴定突跃范围(△pCI): pc+3~pKp-pc”-3 ·影响突跃的因素: 40 sp 0 3 ~ pK 3 sp sp • 滴定突跃范围(ΔpCl ): pc pc + − − • 影响突跃的因素: 0 ; sp c K 1 (3) [ ] [ ] − + − sp点 Cl = Ag = Ks p mol L 0 0 0 0 [ ] c V c V Ag V V + − = + 0 3 3 0 [ ] 10 10 2 c sp Ag c + − − = = 1 0 1 1 a c mol L a − − = + 0 3 sp pCl pK pc = − − sp 当 E a r = + = 0.1%, 100.1% 时: [ ] [ ] K sp Cl Ag − + = (4) sp后 (V>V0 ) 4