正在加载图片...
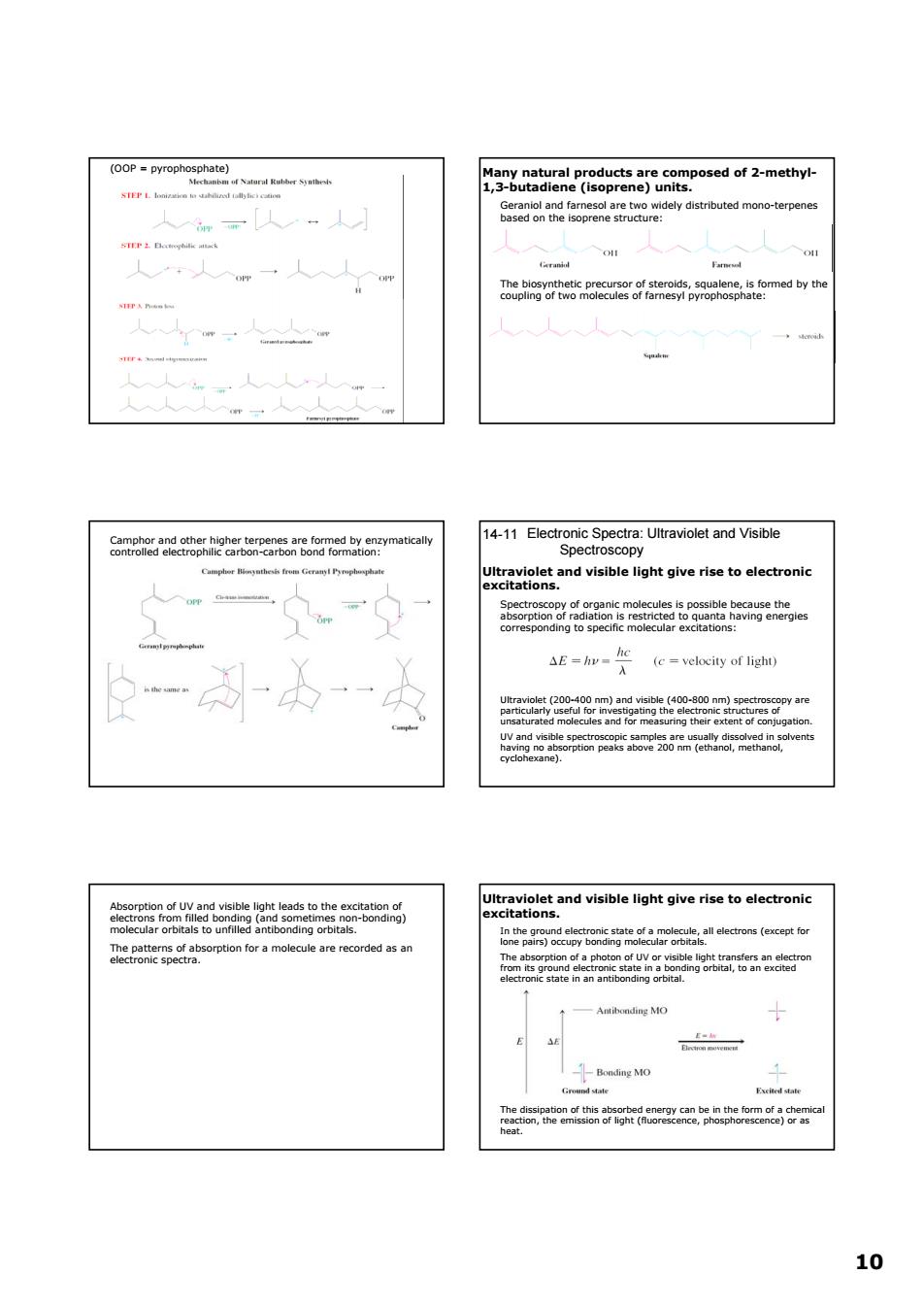
tributed mono-ter 人m[人人 Seademeteopeaarieiet 人人 m一人人人 g52aomedy ·人人 Omehea2dogrh能e2raPen5arcoeaTearem nEnagsaeooeave a22 E(c-velocity of light) a travoeand visible ligh giveristoni mteodireateaobeeedom(eacete E 1010 (OOP = pyrophosphate) Many natural products are composed of 2-methyl- 1,3-butadiene (isoprene) units. Geraniol and farnesol are two widely distributed mono-terpenes based on the isoprene structure: The biosynthetic precursor of steroids, squalene, is formed by the coupling of two molecules of farnesyl pyrophosphate: Camphor and other higher terpenes are formed by enzymatically controlled electrophilic carbon-carbon bond formation: Electronic Spectra: Ultraviolet and Visible Spectroscopy 14-11 Ultraviolet and visible light give rise to electronic excitations. Spectroscopy of organic molecules is possible because the absorption of radiation is restricted to quanta having energies corresponding to specific molecular excitations: Ultraviolet (200-400 nm) and visible (400-800 nm) spectroscopy are particularly useful for investigating the electronic structures of unsaturated molecules and for measuring their extent of conjugation. UV and visible spectroscopic samples are usually dissolved in solvents having no absorption peaks above 200 nm (ethanol, methanol, cyclohexane). Absorption of UV and visible light leads to the excitation of electrons from filled bonding (and sometimes non-bonding) molecular orbitals to unfilled antibonding orbitals. The patterns of absorption for a molecule are recorded as an electronic spectra. Ultraviolet and visible light give rise to electronic excitations. In the ground electronic state of a molecule, all electrons (except for lone pairs) occupy bonding molecular orbitals. The absorption of a photon of UV or visible light transfers an electron from its ground electronic state in a bonding orbital, to an excited electronic state in an antibonding orbital. The dissipation of this absorbed energy can be in the form of a chemical reaction, the emission of light (fluorescence, phosphorescence) or as heat