正在加载图片...
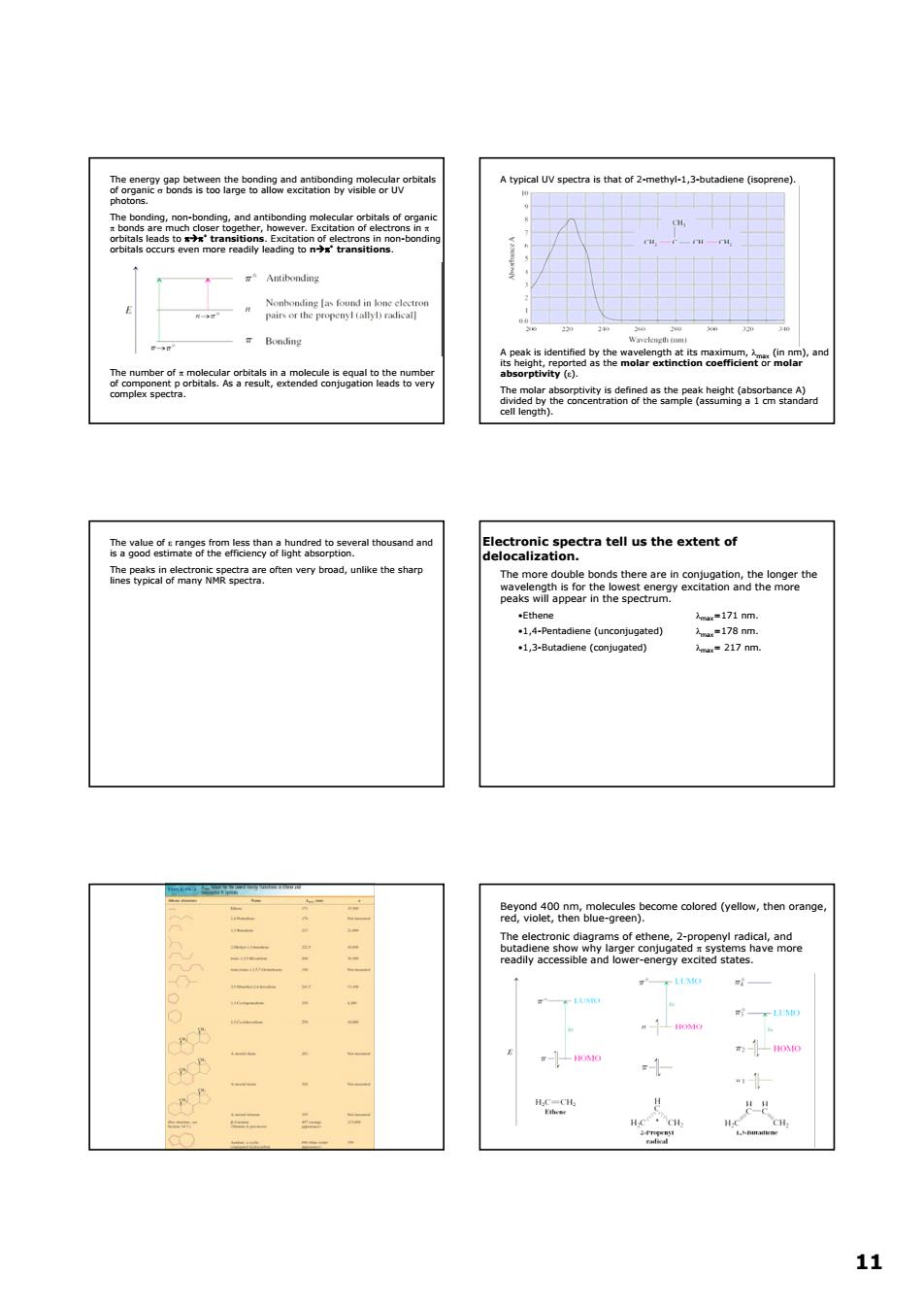
A typical UV spectra is that of 2-methyl-1,3-b B. The tell us the extent of ines typlcal of many ectra anlike the sharp caa 时 1 11 11 The energy gap between the bonding and antibonding molecular orbitals of organic σ bonds is too large to allow excitation by visible or UV photons. The bonding, non-bonding, and antibonding molecular orbitals of organic π bonds are much closer together, however. Excitation of electrons in π orbitals leads to πÆπ* transitions. Excitation of electrons in non-bonding orbitals occurs even more readily leading to nÆπ* transitions. The number of π molecular orbitals in a molecule is equal to the number of component p orbitals. As a result, extended conjugation leads to very complex spectra. A typical UV spectra is that of 2-methyl-1,3-butadiene (isoprene). A peak is identified by the wavelength at its maximum, λmax (in nm), and its height, reported as the molar extinction coefficient or molar absorptivity (ε). The molar absorptivity is defined as the peak height (absorbance A) divided by the concentration of the sample (assuming a 1 cm standard cell length). The value of ε ranges from less than a hundred to several thousand and is a good estimate of the efficiency of light absorption. The peaks in electronic spectra are often very broad, unlike the sharp lines typical of many NMR spectra. Electronic spectra tell us the extent of delocalization. The more double bonds there are in conjugation, the longer the wavelength is for the lowest energy excitation and the more peaks will appear in the spectrum. •Ethene λmax=171 nm. •1,4-Pentadiene (unconjugated) λmax=178 nm. •1,3-Butadiene (conjugated) λmax= 217 nm. Beyond 400 nm, molecules become colored (yellow, then orange, red, violet, then blue-green). The electronic diagrams of ethene, 2-propenyl radical, and butadiene show why larger conjugated π systems have more readily accessible and lower-energy excited states