正在加载图片...
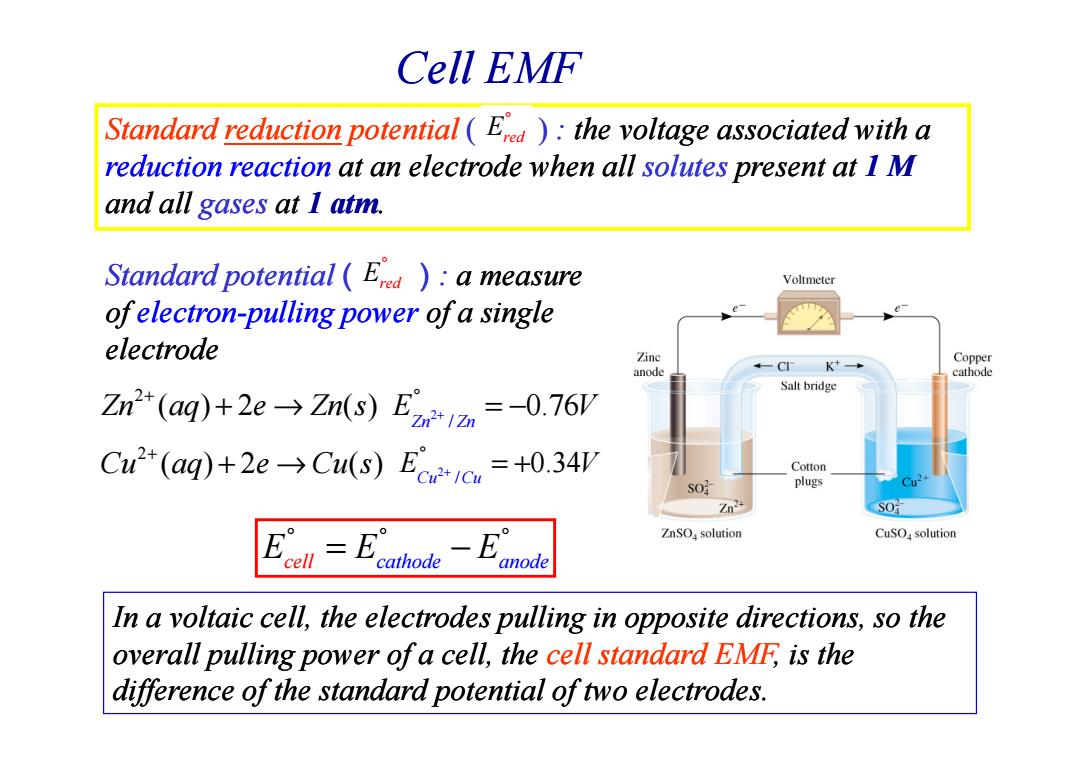
Cell EMF Standard reduction potential (Era):the voltage associated with a reduction reaction at an electrode when all solutes present at I M and all gases at I atm. Standard potential (Erd )a measure Voltmeter ofelectron-pulling power of a single electrode Zinc anode CI K Copper cathode Salt bridge Zn"(aq)+2eZn(s)E=-0.76V Cu(aq)+2eCu(s)Eo=+0.34V Cotton plugs Zn2 SO =B -E° ZnSOa solution CuSOa solution anode In a voltaic cell,the electrodes pulling in opposite directions,so the overall pulling power of a cell,the cell standard EMF,is the difference of the standard potential of two electrodes.Cell EMF Standard Standard reduction reduction potential ( ) : the voltage associated with a reduction reaction at an electrode when all solutes present at 1 M and all gases at 1 atm. Standard potential ( ) : a measure of electron electron-pulling power pulling power of a single electrode Ered ° Ered ° In a voltaic cell, the electrodes pulling in opposite directions, so the overall pulling power of a cell, the cell standard EMF, is the difference of the standard potential of two electrodes. E E E cell cathode anode ° ° ° = − 2 Zn aq e Zn s ( ) 2 ( ) + + → 2 Cu aq e Cu s ( ) 2 ( ) + + → 2 / 0.76 Zn Zn E V + ° = − 2 / 0.34 Cu Cu E V + ° = +