正在加载图片...
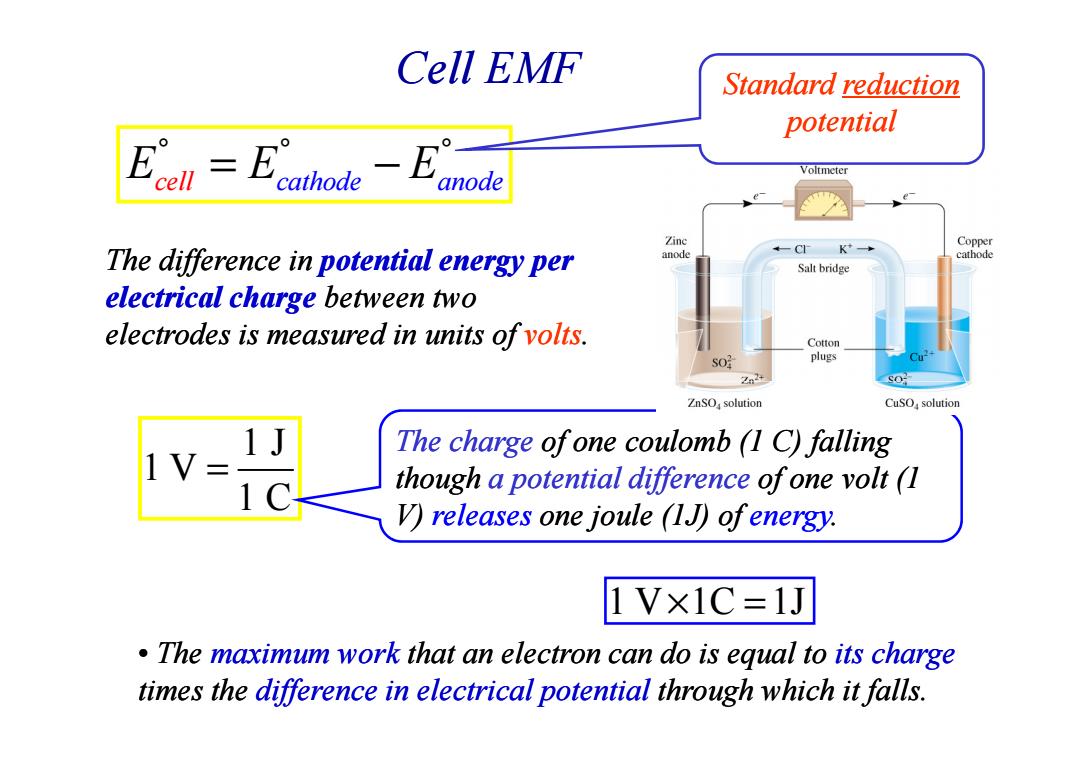
Cell EMF Standard reduction potential Voltmeter anode Zinc The difference in potential energy per anode K Copper cathode Salt bridge electrical charge between two electrodes is measured in units of volts. Cotton SO plugs 224 ZnSOsolution CuSO,solution The charge ofone coulomb (1 C)falling though a potential difference of one volt (1 V)releases one joule (1J)ofenergy. 1V×1C=1J The maximum work that an electron can do is equal to its charge times the difference in electrical potential through which it falls.Cell EMF The difference in potential energy potential energy per electrical charge between two electrodes is measured in units of volts. E E E cell cathode anode ° ° ° = − Standard Standard reduction reduction potential 1 J 1 V 1 C = The charge of one coulomb (1 C) falling though a potential difference of one volt (1 V) releases one joule (1J) of energy. • The maximum work that an electron can do is equal to its charge times the difference in electrical potential through which it falls. 1 V 1C 1J × =