正在加载图片...
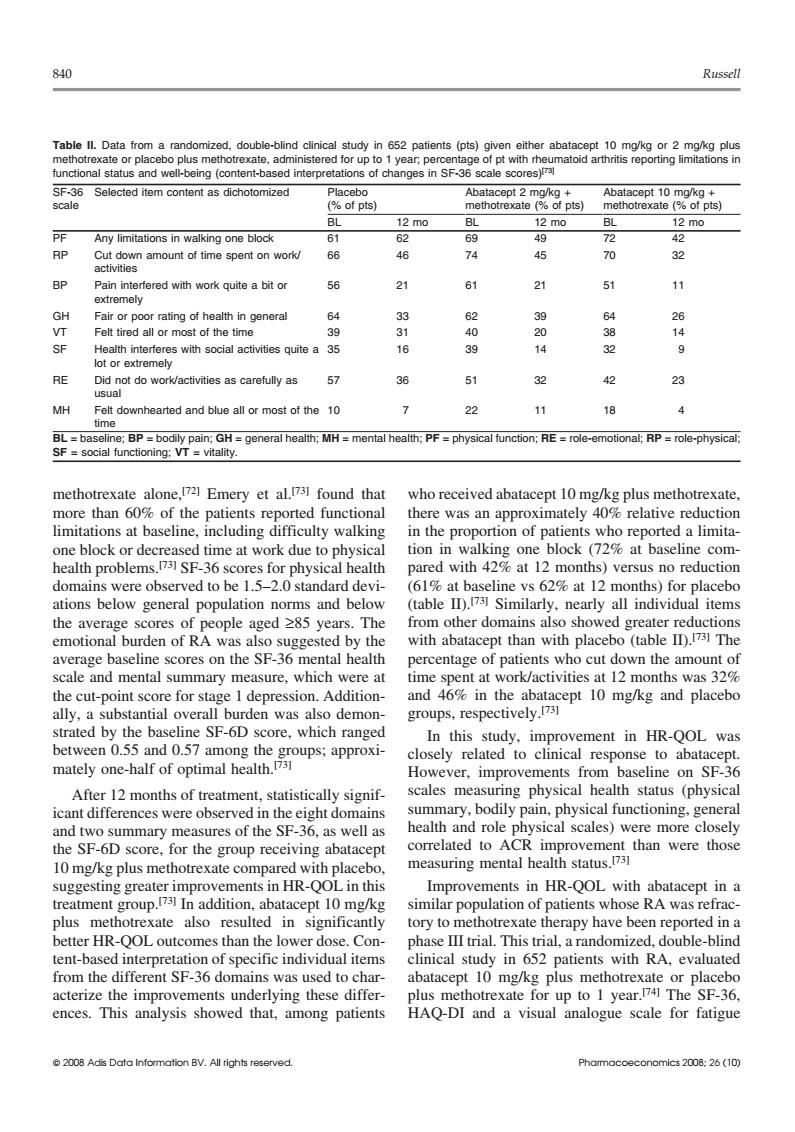
840 Russell Table ll.Dat double-blind clini o plus met ered for upt of pts) 12m0 PE Any limitations in walking ooe block RP amount of 66 46 74 45 70 32 BP Pain in fered with work quite a bit or 56 21 21 51 Fair or poor rating of health in general 33 63 39 64 Felt tired all or most of the tim 40 20 ctivities quite a 35 39 RE 57 36 51 32 42 23 MH Fe downhearted and blue all or most of the10 7 22 11 18 4 than 6 of the 01 culty a limit (729 7sF-36 e at red with 42%at 12 months)versus no reduction domains were observed to be 1.5-2.0 standard devi- (61%at baseline vs 62%at 12 months)for placebo ations below general population norms and below (table ID.Similarly,nearly all individual items the average scores of people aged 285 years.The from other domains also showed greater reductions emotional burden of RA was also suggested by the with abatacept than with placebo (table ID).73 The Dercentage of patients who cut down the amount o and mental summary m sure,which were at t 12 months was 5 cut-p for s epres no SE-6D was which demon and placeb In this study.improvement in HR-QOL was mately n-half between 0.55 and 0.57 among the response After 12 months of treatment,statistically signif S wel health and role acep correlated to ACR improvement than were those measuring mental health status.731 nts in HR-OOLi ments in HR-QOL with abatacep pt in a treatment g up.In addition.abatac 10m ents whose ra was refrac plus methotrexate also resulted in significantly tory to methotrexate therany have been reported in a better HR-QOL outcomes than the lower dose.Con- phase III trial.This trial,a randomized,double-blind tent-based interpret on of specific individual items clinical study in 652 patients with RA,evaluated from the different SF-36 dom uns was used to c actenze up to I year ences. tha rlying these differ among patients HAQ-DI analogue scale for fatigue -ho840 Russell Table II. Data from a randomized, double-blind clinical study in 652 patients (pts) given either abatacept 10 mg/kg or 2 mg/kg plus methotrexate or placebo plus methotrexate, administered for up to 1 year; percentage of pt with rheumatoid arthritis reporting limitations in functional status and well-being (content-based interpretations of changes in SF-36 scale scores)[73] SF-36 Selected item content as dichotomized Placebo Abatacept 2 mg/kg + Abatacept 10 mg/kg + scale (% of pts) methotrexate (% of pts) methotrexate (% of pts) BL 12 mo BL 12 mo BL 12 mo PF Any limitations in walking one block 61 62 69 49 72 42 RP Cut down amount of time spent on work/ 66 46 74 45 70 32 activities BP Pain interfered with work quite a bit or 56 21 61 21 51 11 extremely GH Fair or poor rating of health in general 64 33 62 39 64 26 VT Felt tired all or most of the time 39 31 40 20 38 14 SF Health interferes with social activities quite a 35 16 39 14 32 9 lot or extremely RE Did not do work/activities as carefully as 57 36 51 32 42 23 usual MH Felt downhearted and blue all or most of the 10 7 22 11 18 4 time BL = baseline; BP = bodily pain; GH = general health; MH = mental health; PF = physical function; RE = role-emotional; RP = role-physical; SF = social functioning; VT = vitality. methotrexate alone,[72] Emery et al.[73] found that who received abatacept 10 mg/kg plus methotrexate, more than 60% of the patients reported functional there was an approximately 40% relative reduction limitations at baseline, including difficulty walking in the proportion of patients who reported a limitaone block or decreased time at work due to physical tion in walking one block (72% at baseline comhealth problems. pared with 42% at 12 months) versus no reduction [73] SF-36 scores for physical health domains were observed to be 1.5–2.0 standard devi- (61% at baseline vs 62% at 12 months) for placebo (table II).[73] ations below general population norms and below Similarly, nearly all individual items the average scores of people aged ≥85 years. The from other domains also showed greater reductions with abatacept than with placebo (table II).[73] emotional burden of RA was also suggested by the The average baseline scores on the SF-36 mental health percentage of patients who cut down the amount of scale and mental summary measure, which were at time spent at work/activities at 12 months was 32% the cut-point score for stage 1 depression. Addition- and 46% in the abatacept 10 mg/kg and placebo groups, respectively.[73] ally, a substantial overall burden was also demonstrated by the baseline SF-6D score, which ranged In this study, improvement in HR-QOL was between 0.55 and 0.57 among the groups; approxi- closely related to clinical response to abatacept. mately one-half of optimal health.[73] However, improvements from baseline on SF-36 After 12 months of treatment, statistically signif- scales measuring physical health status (physical summary, bodily pain, physical functioning, general icant differences were observed in the eight domains health and role physical scales) were more closely and two summary measures of the SF-36, as well as correlated to ACR improvement than were those the SF-6D score, for the group receiving abatacept measuring mental health status.[73] 10 mg/kg plus methotrexate compared with placebo, suggesting greater improvements in HR-QOL in this Improvements in HR-QOL with abatacept in a treatment group.[73] In addition, abatacept 10 mg/kg similar population of patients whose RA was refracplus methotrexate also resulted in significantly tory to methotrexate therapy have been reported in a better HR-QOL outcomes than the lower dose. Con- phase III trial. This trial, a randomized, double-blind tent-based interpretation of specific individual items clinical study in 652 patients with RA, evaluated from the different SF-36 domains was used to char- abatacept 10 mg/kg plus methotrexate or placebo acterize the improvements underlying these differ- plus methotrexate for up to 1 year.[74] The SF-36, ences. This analysis showed that, among patients HAQ-DI and a visual analogue scale for fatigue © 2008 Adis Data Information BV. All rights reserved. Pharmacoeconomics 2008; 26 (10)