正在加载图片...
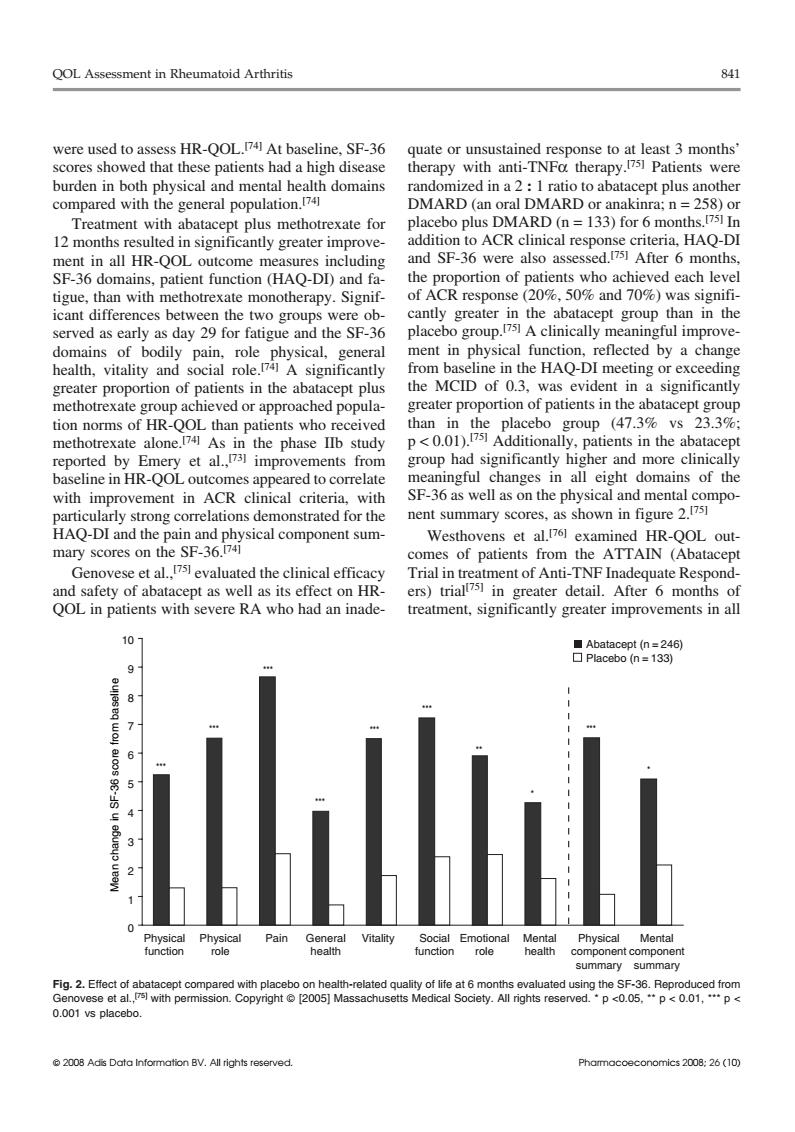
QOL Assessment in Rheumatoid Arthritis 841 were used to assess HR-QOL.74 At baseline.SF-36 te or scores showed that these patients had a high disease burden in both physical and mental health domains compared with the general population.74 DMARD (an oral DMARD or anakinra;n=258)or Treatment with abatacep plus methotrexate for placebo plus DMARD (n=133)for 6 months.!751 In 12 months resulted in significantly greater improve- S6Roo were s da 29a d the SF nent in physical function. reflected by a cha from baseline in the HAQ-DI meeting or exceeding greater proportion of patients in the abatacept plus the MCID of 0.3,was evident in a significantly methotrexate group achieved or approached popula- greater proportion of patients in the abatacept group tion norms of HR-QOL than patients who received than group (47.3% 23.3% methotrexate alone." As p<0.0i patients in the nents From ore clinic a i油 in A SF-36 as well as on the physical and mental c HAO-DL and the nd mponent sum Westh HR-OOL out se et al isl evaluated the clinical efficad and safety of abatacept as well as its effect on HR- ers)trials in greater detail.After 6 months of QOL in patients with severe RA who had an inade- treatment,significantly greater improvements in all 10 ublb Pain ent compone 0.001 vs placebo 2008 Adis Data Information BV.All rights resorved Phamocooconomics 2008:26 (10) QOL Assessment in Rheumatoid Arthritis 841 were used to assess HR-QOL.[74] At baseline, SF-36 quate or unsustained response to at least 3 months’ scores showed that these patients had a high disease therapy with anti-TNFα therapy.[75] Patients were burden in both physical and mental health domains randomized in a 2 : 1 ratio to abatacept plus another compared with the general population.[74] DMARD (an oral DMARD or anakinra; n = 258) or placebo plus DMARD (n = 133) for 6 months.[75] Treatment with abatacept plus methotrexate for In 12 months resulted in significantly greater improve- addition to ACR clinical response criteria, HAQ-DI and SF-36 were also assessed.[75] ment in all HR-QOL outcome measures including After 6 months, SF-36 domains, patient function (HAQ-DI) and fa- the proportion of patients who achieved each level tigue, than with methotrexate monotherapy. Signif- of ACR response (20%, 50% and 70%) was signifiicant differences between the two groups were ob- cantly greater in the abatacept group than in the placebo group.[75] served as early as day 29 for fatigue and the SF-36 A clinically meaningful improvedomains of bodily pain, role physical, general ment in physical function, reflected by a change health, vitality and social role.[74] A significantly from baseline in the HAQ-DI meeting or exceeding greater proportion of patients in the abatacept plus the MCID of 0.3, was evident in a significantly methotrexate group achieved or approached popula- greater proportion of patients in the abatacept group tion norms of HR-QOL than patients who received than in the placebo group (47.3% vs 23.3%; p < 0.01).[75] methotrexate alone. Additionally, patients in the abatacept [74] As in the phase IIb study reported by Emery et al.,[73] improvements from group had significantly higher and more clinically baseline in HR-QOL outcomes appeared to correlate meaningful changes in all eight domains of the with improvement in ACR clinical criteria, with SF-36 as well as on the physical and mental component summary scores, as shown in figure 2.[75] particularly strong correlations demonstrated for the HAQ-DI and the pain and physical component sum- Westhovens et al.[76] examined HR-QOL outmary scores on the SF-36.[74] comes of patients from the ATTAIN (Abatacept Genovese et al.,[75] evaluated the clinical efficacy Trial in treatment of Anti-TNF Inadequate Respondand safety of abatacept as well as its effect on HR- ers) trial[75] in greater detail. After 6 months of QOL in patients with severe RA who had an inade- treatment, significantly greater improvements in all 0 1 2 3 4 5 6 7 8 9 10 Physical function Mean change in SF-36 score from baseline Physical role Pain General health Vitality Social function Emotional role Mental health Physical component summary Mental component summary Abatacept (n = 246) Placebo (n = 133) ** *** * *** *** *** *** *** *** * Fig. 2. Effect of abatacept compared with placebo on health-related quality of life at 6 months evaluated using the SF-36. Reproduced from Genovese et al.,[75] with permission. Copyright © [2005] Massachusetts Medical Society. All rights reserved. * p <0.05, ** p < 0.01, *** p < 0.001 vs placebo. © 2008 Adis Data Information BV. All rights reserved. Pharmacoeconomics 2008; 26 (10)