正在加载图片...
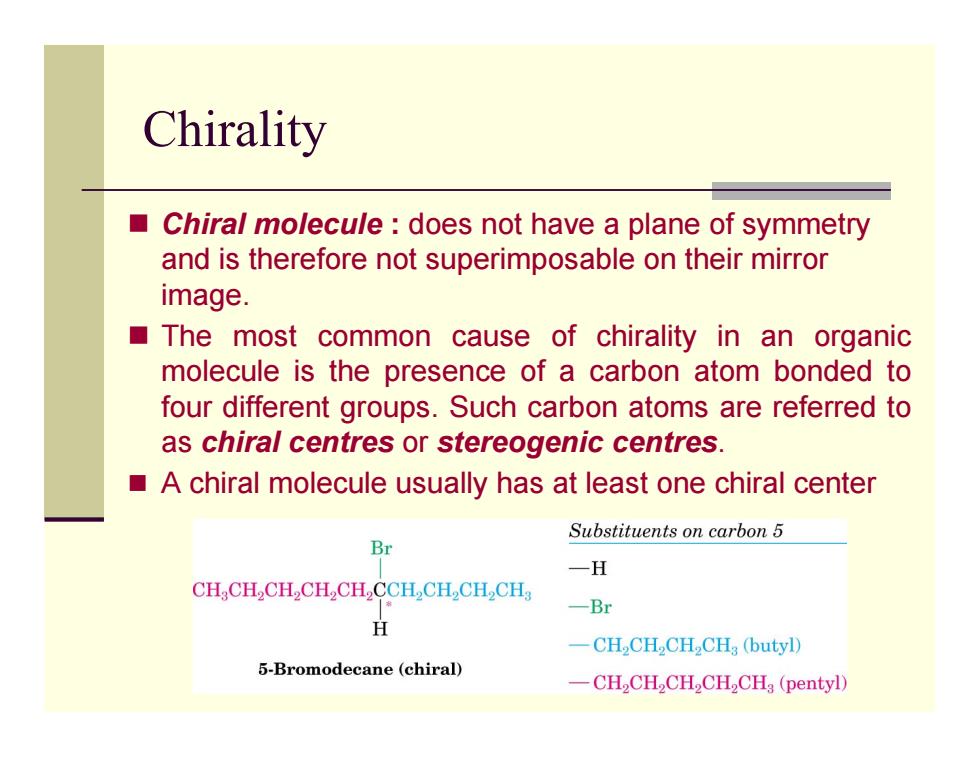
Chirality ■ Chiral molecule does not have a plane of symmetry and is therefore not superimposable on their mirror image. The most common cause of chirality in an organic molecule is the presence of a carbon atom bonded to four different groups.Such carbon atoms are referred to as chiral centres or stereogenic centres. A chiral molecule usually has at least one chiral center Substituents on carbon 5 Br 一H CHCH2CH,CH2CH,CCH,CH,CH2CH 一Br H 一CH2CHCH2CH3(butyl) 5-Bromodecane (chiral) -CH2CH2CH2CH2CHg(pentyl)Chirality Chiral molecule : does not have a plane of symmetry and is therefore not superimposable on their mirror image. The most common cause of chirality in an organic molecule is the presence of a carbon atom bonded to four different groups. Such carbon atoms are referred to as chiral centres or stereogenic centres. A chiral molecule usually has at least one chiral center