正在加载图片...
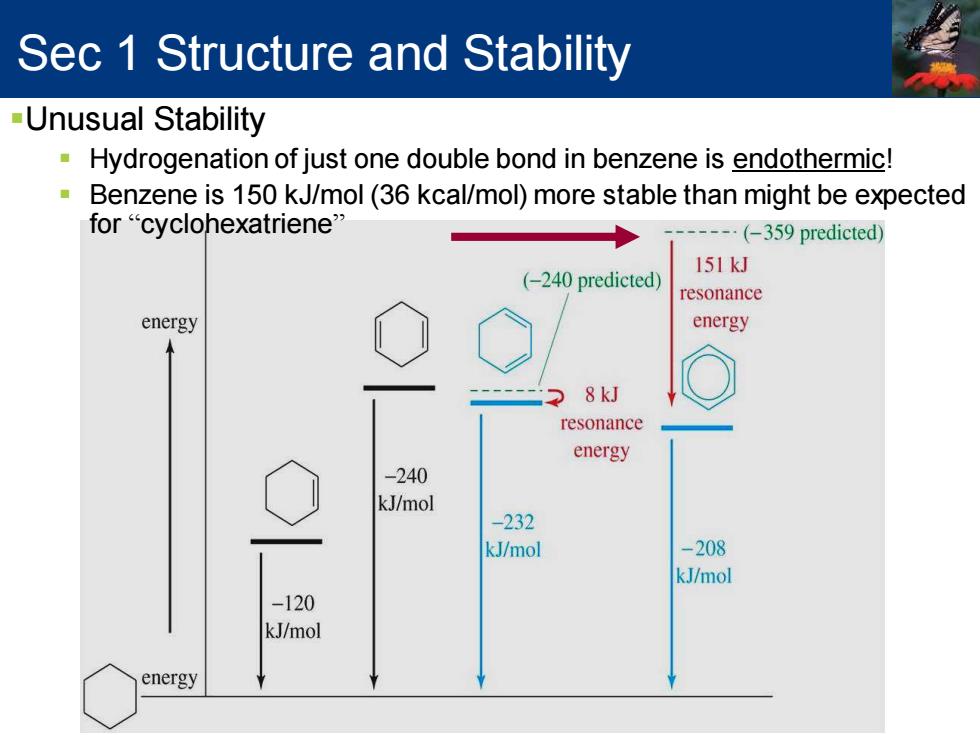
Sec 1 Structure and Stability -Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! Benzene is 150 kJ/mol(36 kcal/mol)more stable than might be expected for "cyclohexatriene" (-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energySec 1 Structure and Stability ▪Unusual Stability ▪ Hydrogenation of just one double bond in benzene is endothermic! ▪ Benzene is 150 kJ/mol (36 kcal/mol) more stable than might be expected for “cyclohexatriene