正在加载图片...
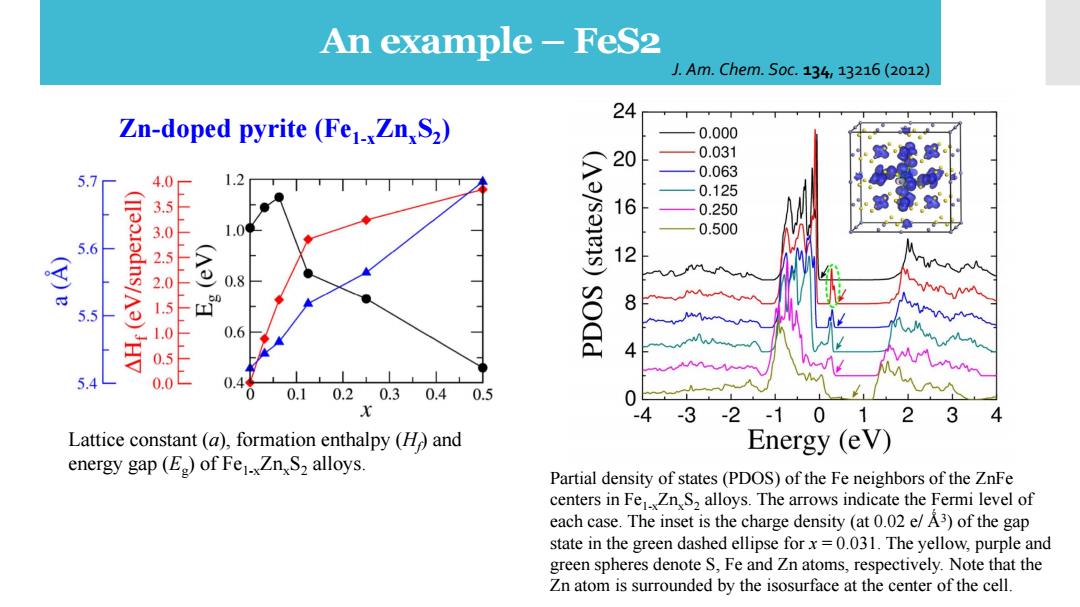
An example-FeS2 J.Am.Chem.S0c.134,13216(2012) 24 Zn-doped pyrite (Fe1xZn,S2) 0.000 0.031 5.7 4.0 20 0.063 0.125 1 0.250 0.500 5.6 12 0.8 8 5.5 500150150 0.6 sodd 0.0 0.44 0.10.20.3 0.40.5 x 3 -2-10 12 3 4 Lattice constant (a),formation enthalpy (H and Energy (eV) energy gap(E)of Fe1.Zn S2 alloys. Partial density of states(PDOS)of the Fe neighbors of the ZnFe centers in FeZnS2 alloys.The arrows indicate the Fermi level of each case.The inset is the charge density (at 0.02 e/A3)of the gap state in the green dashed ellipse forx=0.031.The yellow,purple and green spheres denote S,Fe and Zn atoms,respectively.Note that the Zn atom is surrounded by the isosurface at the center of the cell.An example – FeS2 Partial density of states (PDOS) of the Fe neighbors of the ZnFe centers in Fe1-xZnxS2 alloys. The arrows indicate the Fermi level of each case. The inset is the charge density (at 0.02 e/ Ǻ3 ) of the gap state in the green dashed ellipse for x = 0.031. The yellow, purple and green spheres denote S, Fe and Zn atoms, respectively. Note that the Zn atom is surrounded by the isosurface at the center of the cell. J. Am. Chem. Soc. 134, 13216 (2012) Lattice constant (a), formation enthalpy (Hf ) and energy gap (Eg ) of Fe1-xZnxS2 alloys. Zn-doped pyrite (Fe1-xZnxS2 )