正在加载图片...
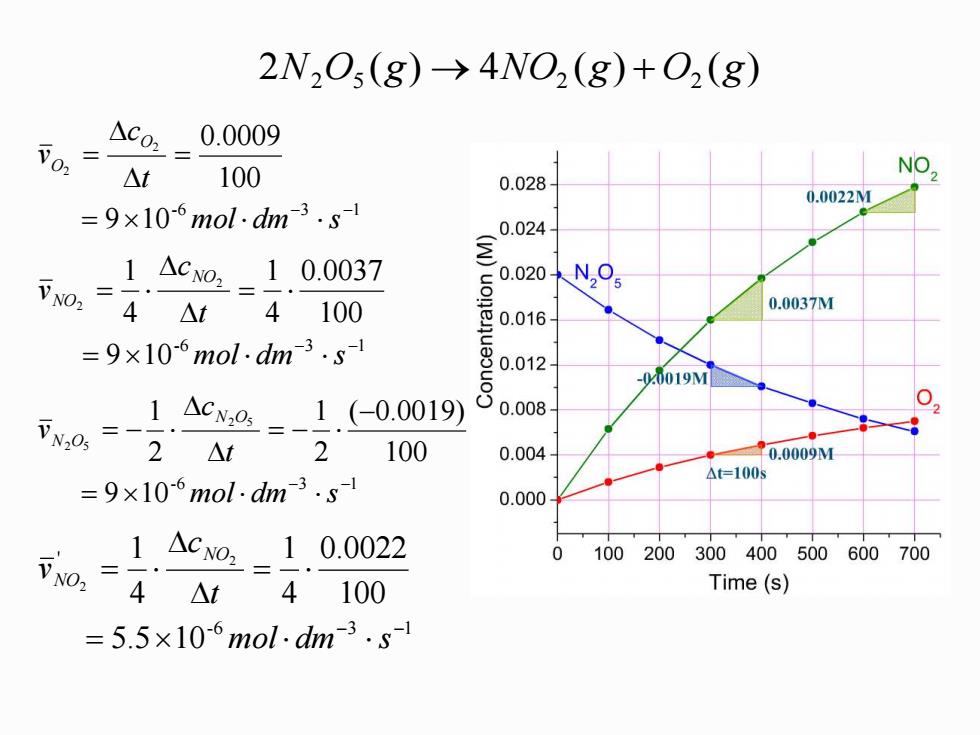
2N,Os(g)->4NO,(g)+0,(g) 0, △co,_0.0009 △t 100 NO 0.028 0.0022M =9×10-6mol.dhm3.s 0.024 1.△c02-1.0.0037 V02= 4△t 4100 0.0037M 0.016 =9×10-6mol.dm3.sl 00m2 00081 -0.0019M N0,= -1.Acx@=-1.(-0.0019) 2 △t 100 0.004 0.0009M △=1005 =9×l0-6mol:dm3.s 0.000 1△co2-10.0022 0100200300400500600700 可o,=4At 4100 Time(s) =5.5×106mol-dm3.s1 2 ( ) 4 ( ) ( ) N2 O5 g → NO2 g + O2 g -6 3 1 9 10 100 2 0.0009 2 − − = = = mol dm s t c v O O -6 3 1 9 10 100 0.0037 4 1 4 1 2 2 − − = = = mol dm s t c v NO NO -6 3 1 9 10 100 ( 0.0019) 2 1 2 1 2 5 2 5 − − = − = − = − mol dm s t c v N O N O -6 3 1 ' 5.5 10 100 0.0022 4 1 4 1 2 2 − − = = = mol dm s t c v NO NO