正在加载图片...
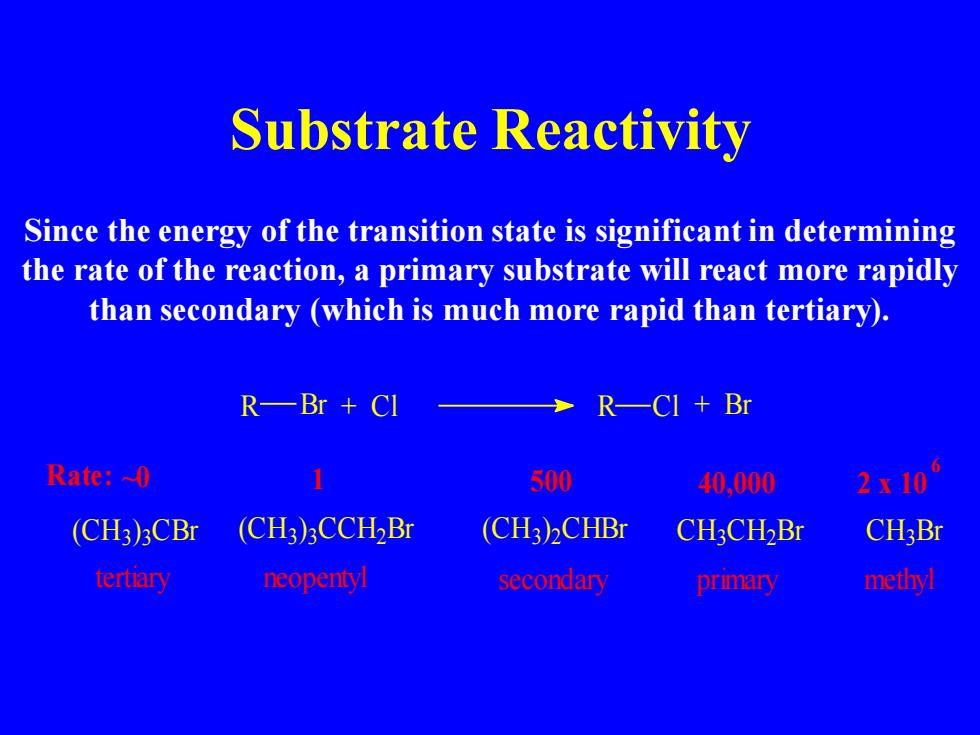
Substrate Reactivity Since the energy of the transition state is significant in determining the rate of the reaction,a primary substrate will react more rapidly than secondary (which is much more rapid than tertiary). R—Br+CI R一CI+Br Rate:~0 500 40,000 2x10 (CH3)3CBr (CH3)3CCH2Br (CH3)2CHBr CH3CH2Br CH3Br tertiary neopenty secondary primary methyl Substrate Reactivity Since the energy of the transition state is significant in determining the rate of the reaction, a primary substrate will react more rapidly than secondary (which is much more rapid than tertiary). 6 tertiary neopentyl secondary primary methyl Rate: ~0 (CH3 ) 3 CBr C H C H3 Br (CH3) 3CCH2Br (CH3) 2CHBr 3 C H2 Br R Br + Cl R Cl + Br 1 500 40,000 2 x 10