正在加载图片...
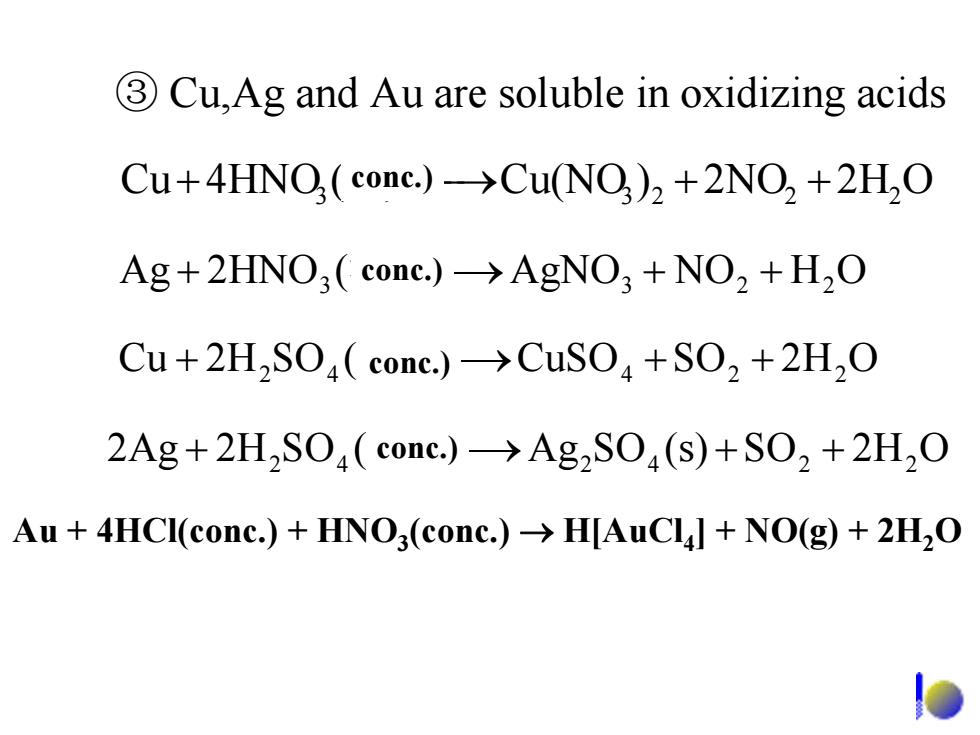
3Cu,Ag and Au are soluble in oxidizing acids Cu+4HNO(cone.)->Cu(NO)2+2NO2 +2H2O Ag+2HNO3 cone.)->AgNO3 +NO2 +H2O Cu+2H2SO(cone.)->CuSO+SO2+2H2O 2Ag+2H2SO(conc.)->Ag2SO(s)+SO2+2H2O Au 4HCI(conc.)+HNO3(conc.)>H[AuCll NO(g)+2H2O ③ Cu,Ag and Au are soluble in oxidizing acids + 3 浓)(4HNOCu ⎯⎯→ 23 ++ 22 O2H2NO)Cu(NO O2HSO(s)SOAg)(SO2H2Ag + 42 浓 ⎯⎯→ 42 ++ 22 + 42 浓)(SO2HCu ⎯⎯→CuSO ++ 224 O2HSO + 3 浓)(2HNOAg ⎯⎯→AgNO3 ++ 22 OHNO conc.) conc.) conc.) conc.) Au + 4HCl(conc.) + HNO3(conc.) → H[AuCl4] + NO(g) + 2H2O