正在加载图片...
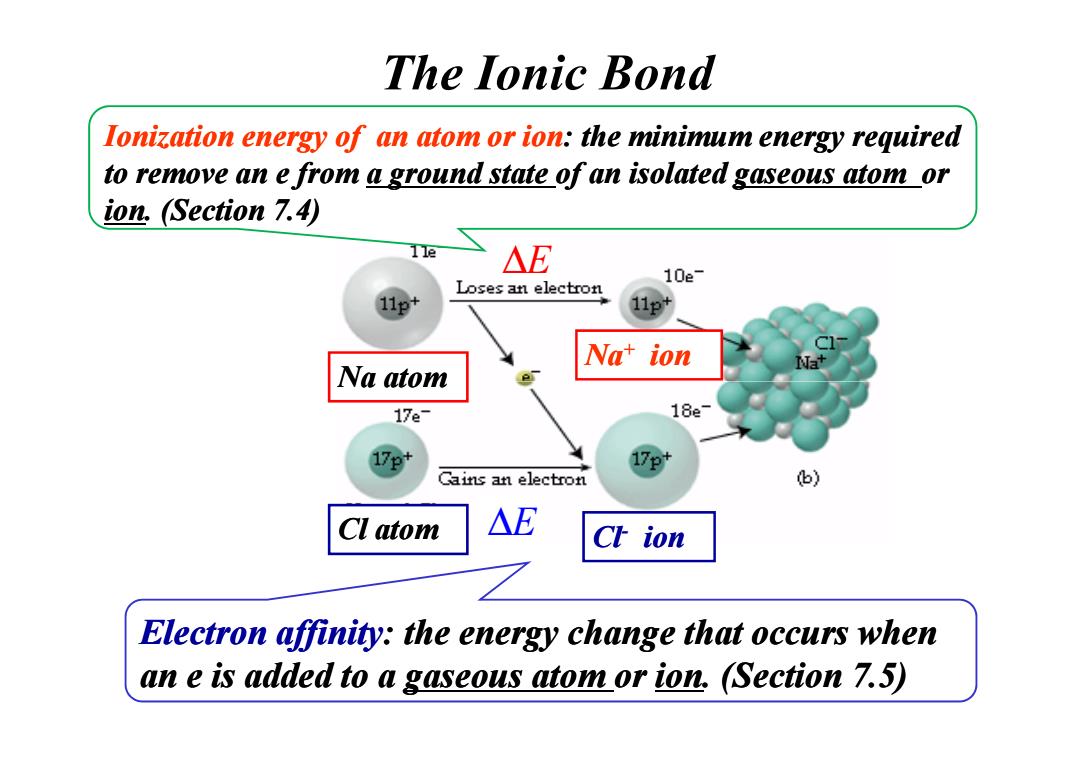
The lonic bond lonization energy of an atom or ion:the minimum energy required to remove an e from a ground state of an isolated gaseous atom or ion.(Section 7.4) 11e △E 10e- Loses an electron 11p+ Nat ion Na atom 17e- 18e 17e* Cains an electron 6) Cl atom △E Ch ion Electron affinity:the energy change that occurs when an e is added to a gaseous atom or ion,(Section 7.5)Na atom Na+ ion Ionization energy of an atom or ion: the minimum energy required to remove an e from to remove an e from a ground state a ground state of an isolated of an isolated gaseous atom gaseous atom or ion. (Section 7.4) The Ionic Bond ∆E Na atom Cl- Cl atom ion Electron affinity: the energy change that occurs when an e is added to a an e is added to a gaseous atom gaseous atom or ion. (Section 7.5) ∆E