正在加载图片...
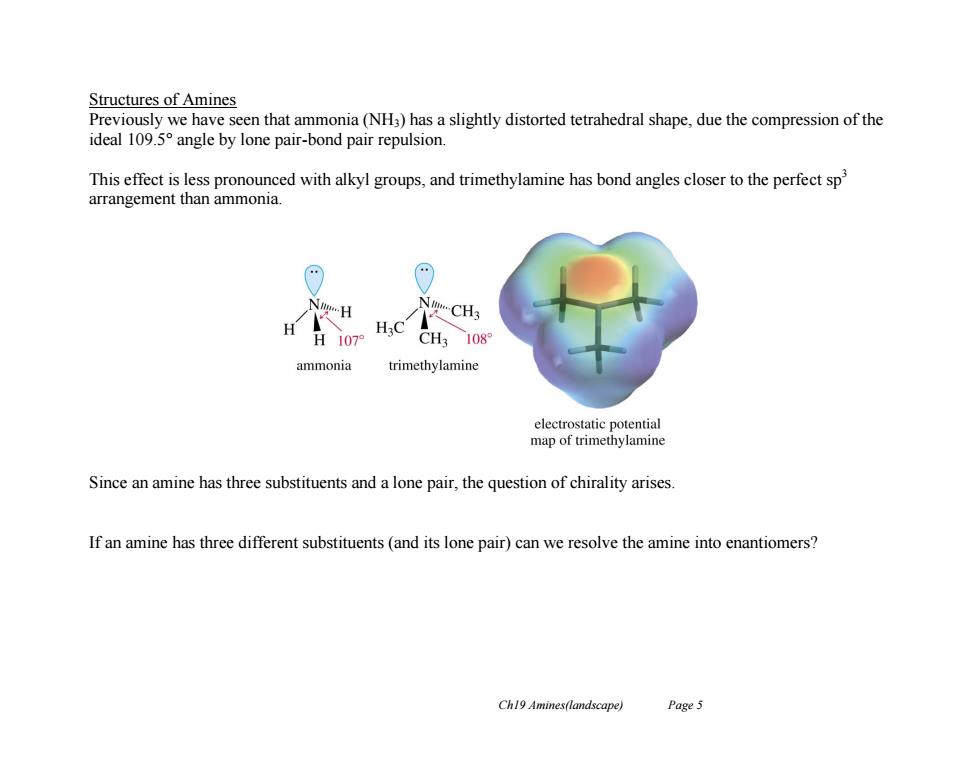
Structures of Amines Previously we have seen that ammonia(NH3)has a slightly distorted tetrahedral shape,due the compression of the ideal 109.5 angle by lone pair-bond pair repulsion. This effect is less pronounced with alkyl groups,and trimethylamine has bond angles closer to the perfect sp arrangement than ammonia. NgH N-CH3 H107 CH3 108 ammonia trimethylamine electrostatic potential map of trimethylamine Since an amine has three substituents and a lone pair,the question of chirality arises. If an amine has three different substituents(and its lone pair)can we resolve the amine into enantiomers? Ch19 Amines(landscape) Page 5Ch19 Amines(landscape) Page 5 Structures of Amines Previously we have seen that ammonia (NH3) has a slightly distorted tetrahedral shape, due the compression of the ideal 109.5° angle by lone pair-bond pair repulsion. This effect is less pronounced with alkyl groups, and trimethylamine has bond angles closer to the perfect sp3 arrangement than ammonia. Since an amine has three substituents and a lone pair, the question of chirality arises. If an amine has three different substituents (and its lone pair) can we resolve the amine into enantiomers?