正在加载图片...
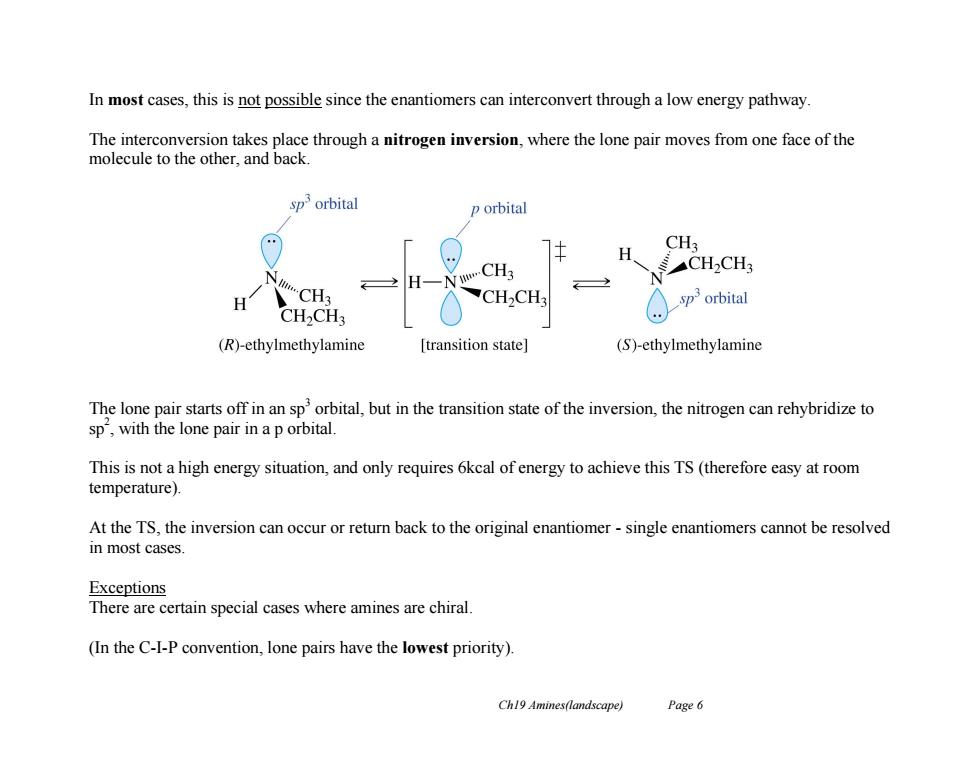
In most cases,this is not possible since the enantiomers can interconvert through a low energy pathway The interconversion takes place through a nitrogen inversion,where the lone pair moves from one face of the molecule to the other,and back sporbital p orbital CH3 CH2CH3 N H CH3 H CH3 CH2CH3 sporbital CH2CH3 (R)-ethylmethylamine [transition state] (S)-ethylmethylamine The lone pair starts off in an sp'orbital,but in the transition state of the inversion,the nitrogen can rehybridize to sp,with the lone pair in a p orbital. This is not a high energy situation,and only requires 6kcal of energy to achieve this TS(therefore easy at room temperature). At the TS,the inversion can occur or return back to the original enantiomer-single enantiomers cannot be resolved in most cases. Exceptions There are certain special cases where amines are chiral. (In the C-I-P convention,lone pairs have the lowest priority). Ch19 Amines(landscape) Page 6 Ch19 Amines(landscape) Page 6 In most cases, this is not possible since the enantiomers can interconvert through a low energy pathway. The interconversion takes place through a nitrogen inversion, where the lone pair moves from one face of the molecule to the other, and back. The lone pair starts off in an sp3 orbital, but in the transition state of the inversion, the nitrogen can rehybridize to sp 2 , with the lone pair in a p orbital. This is not a high energy situation, and only requires 6kcal of energy to achieve this TS (therefore easy at room temperature). At the TS, the inversion can occur or return back to the original enantiomer - single enantiomers cannot be resolved in most cases. Exceptions There are certain special cases where amines are chiral. (In the C-I-P convention, lone pairs have the lowest priority)