正在加载图片...
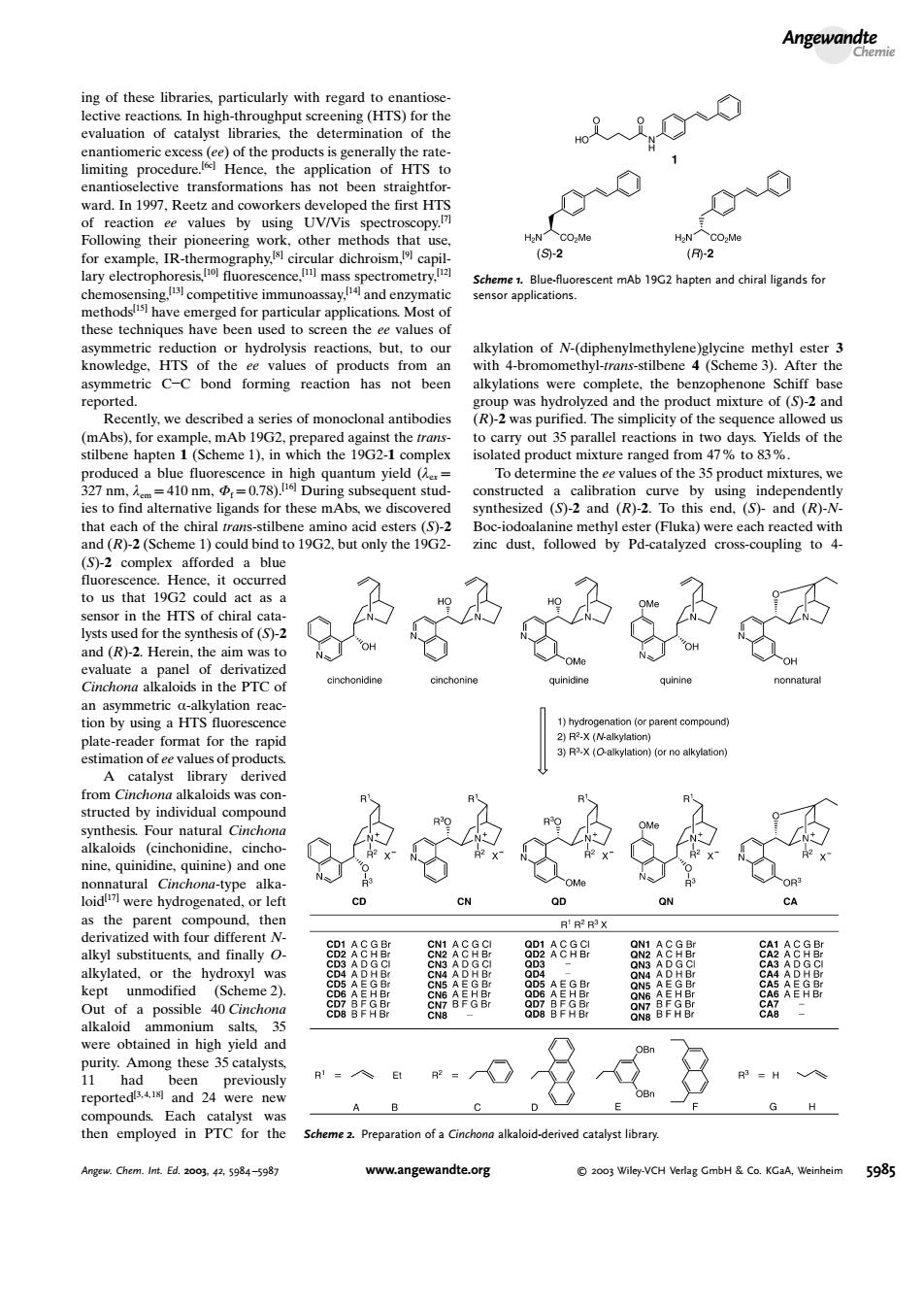
Angewandte ctermir the has ea ee values by using UV/Vis oth ar dic 12 scence mas pectrom etry. Scheme.Bluefuorescent mAb 19G2 hapen and chiral ligands for app nodslisl have emer ed for par icular applicatio to screen the of N-(diph knowledge.HTS of the ee values of pro rming reacton has no eer v.we d scril of monoclo al ant dies R)-2 was p I the se ().in hich the xture m47% 03% 410a nd a (S)-2 and (R) end.(S)-and (RH d (R)-2(Se bind to 2.but only the 19G2 inc dust.followed by Pd-catalvzed cross-coupling to 19G oul lysts u the synthes s of (S)-2 Here lkaloids in the PTC tion by usins a HTS u plate-r the rapi catalyst derive dby individual nin ninea as the parent ther alkyl substituents.and finally O ated.or the A9 Out of a possible Cinchon 8F96 were obtained in high yield and ong thes reported and 24 were nev A B G H akaod-derived 5934 angewandte.org a003 Wiley-VCH bH& 598ing of these libraries, particularly with regard to enantioselective reactions. In high-throughput screening (HTS) for the evaluation of catalyst libraries, the determination of the enantiomeric excess (ee) of the products is generally the ratelimiting procedure.[6c] Hence, the application of HTS to enantioselective transformations has not been straightforward. In 1997, Reetz and coworkers developed the first HTS of reaction ee values by using UV/Vis spectroscopy.[7] Following their pioneering work, other methods that use, for example, IR-thermography,[8] circular dichroism,[9] capillary electrophoresis,[10] fluorescence,[11] mass spectrometry,[12] chemosensing,[13] competitive immunoassay,[14] and enzymatic methods[15] have emerged for particular applications. Most of these techniques have been used to screen the ee values of asymmetric reduction or hydrolysis reactions, but, to our knowledge, HTS of the ee values of products from an asymmetric CC bond forming reaction has not been reported. Recently, we described a series of monoclonal antibodies (mAbs), for example, mAb 19G2, prepared against the transstilbene hapten 1 (Scheme 1), in which the 19G2-1 complex produced a blue fluorescence in high quantum yield (lex = 327 nm, lem = 410 nm, Ff = 0.78).[16] During subsequent studies to find alternative ligands for these mAbs, we discovered that each of the chiral trans-stilbene amino acid esters (S)-2 and (R)-2 (Scheme 1) could bind to 19G2, but only the 19G2- (S)-2 complex afforded a blue fluorescence. Hence, it occurred to us that 19G2could act as a sensor in the HTS of chiral catalysts used for the synthesis of (S)-2 and (R)-2. Herein, the aim was to evaluate a panel of derivatized Cinchona alkaloids in the PTC of an asymmetric a-alkylation reaction by using a HTS fluorescence plate-reader format for the rapid estimation of ee values of products. A catalyst library derived from Cinchona alkaloids was constructed by individual compound synthesis. Four natural Cinchona alkaloids (cinchonidine, cinchonine, quinidine, quinine) and one nonnatural Cinchona-type alkaloid[17] were hydrogenated, or left as the parent compound, then derivatized with four different Nalkyl substituents, and finally Oalkylated, or the hydroxyl was kept unmodified (Scheme 2). Out of a possible 40 Cinchona alkaloid ammonium salts, 35 were obtained in high yield and purity. Among these 35 catalysts, 11 had been previously reported[3, 4, 18] and 24 were new compounds. Each catalyst was then employed in PTC for the alkylation of N-(diphenylmethylene)glycine methyl ester 3 with 4-bromomethyl-trans-stilbene 4 (Scheme 3). After the alkylations were complete, the benzophenone Schiff base group was hydrolyzed and the product mixture of (S)-2 and (R)-2 was purified. The simplicity of the sequence allowed us to carry out 35 parallel reactions in two days. Yields of the isolated product mixture ranged from 47% to 83%. To determine the ee values of the 35 product mixtures, we constructed a calibration curve by using independently synthesized (S)-2 and (R)-2. To this end, (S)- and (R)-NBoc-iodoalanine methyl ester (Fluka) were each reacted with zinc dust, followed by Pd-catalyzed cross-coupling to 4- Scheme 1. Blue-fluorescent mAb 19G2 hapten and chiral ligands for sensor applications. Scheme 2. Preparation of a Cinchona alkaloid-derived catalyst library. Angewandte Chemie Angew. Chem. Int. Ed. 2003, 42, 5984 –5987 www.angewandte.org 2003 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 5985