正在加载图片...
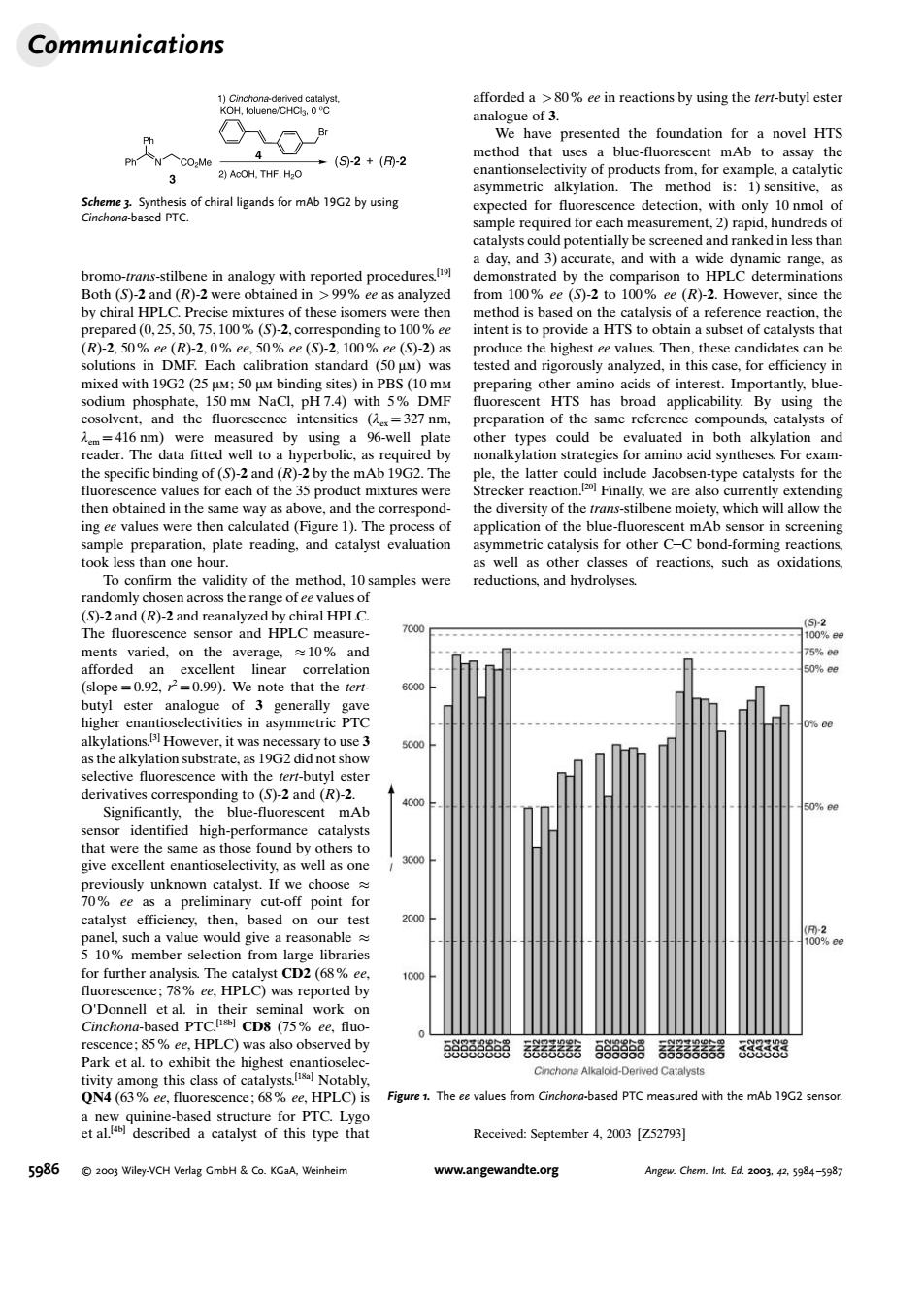
Communications Ko oH 4 (S2+-2 3 m地1c2me apeeqmedoreah a day.and 3)accurate,and with a wide ated by the ther of a reference reac tion. Then the wa ey in 150 mM NaCl,pH7.4)with 5 DME HTS ha 416m %-well p acid synthe xan resc on Finally are als ow th preparation.plate reading.and catalyst evaluation ymmetric c alysis for other CC bond-formnre ction the method.10samples were (S)-2and HPLC The flue sensor and HPLC =0.99 ote that the ter high electivities in mmetric PTO the mAb high-per d h alyst give excellent enantioselectivity,as well as on catalyst the 210% 02 meml :78%e.HPLC)wa O'Donnell on 85%ee HPLC) also observed by QN4 (63%ee.fluc cence:68 ee HPLC)is Figure The evalues from Cinchon-ased PTC measured with the etdescribed a catalyst of this type that Received:September 4.2003 [752793] 5986 ndte.or bromo-trans-stilbene in analogy with reported procedures.[19] Both (S)-2 and (R)-2 were obtained in > 99% ee as analyzed by chiral HPLC. Precise mixtures of these isomers were then prepared (0, 25, 50, 75, 100% (S)-2, corresponding to 100% ee (R)-2, 50% ee (R)-2, 0% ee, 50% ee (S)-2, 100% ee (S)-2) as solutions in DMF. Each calibration standard (50 mm) was mixed with 19G2(25 mm; 50 mm binding sites) in PBS (10 mm sodium phosphate, 150 mm NaCl, pH 7.4) with 5% DMF cosolvent, and the fluorescence intensities (lex = 327 nm, lem = 416 nm) were measured by using a 96-well plate reader. The data fitted well to a hyperbolic, as required by the specific binding of (S)-2 and (R)-2 by the mAb 19G2. The fluorescence values for each of the 35 product mixtures were then obtained in the same way as above, and the corresponding ee values were then calculated (Figure 1). The process of sample preparation, plate reading, and catalyst evaluation took less than one hour. To confirm the validity of the method, 10 samples were randomly chosen across the range of ee values of (S)-2 and (R)-2 and reanalyzed by chiral HPLC. The fluorescence sensor and HPLC measurements varied, on the average, 10% and afforded an excellent linear correlation (slope = 0.92, r 2 = 0.99). We note that the tertbutyl ester analogue of 3 generally gave higher enantioselectivities in asymmetric PTC alkylations.[3] However, it was necessary to use 3 as the alkylation substrate, as 19G2did not show selective fluorescence with the tert-butyl ester derivatives corresponding to (S)-2 and (R)-2. Significantly, the blue-fluorescent mAb sensor identified high-performance catalysts that were the same as those found by others to give excellent enantioselectivity, as well as one previously unknown catalyst. If we choose 70% ee as a preliminary cut-off point for catalyst efficiency, then, based on our test panel, such a value would give a reasonable 5–10% member selection from large libraries for further analysis. The catalyst CD2 (68% ee, fluorescence; 78% ee, HPLC) was reported by O'Donnell et al. in their seminal work on Cinchona-based PTC.[18b] CD8 (75% ee, fluorescence; 85% ee, HPLC) was also observed by Park et al. to exhibit the highest enantioselectivity among this class of catalysts.[18a] Notably, QN4 (63% ee, fluorescence; 68% ee, HPLC) is a new quinine-based structure for PTC. Lygo et al.[4b] described a catalyst of this type that afforded a > 80% ee in reactions by using the tert-butyl ester analogue of 3. We have presented the foundation for a novel HTS method that uses a blue-fluorescent mAb to assay the enantionselectivity of products from, for example, a catalytic asymmetric alkylation. The method is: 1) sensitive, as expected for fluorescence detection, with only 10 nmol of sample required for each measurement, 2) rapid, hundreds of catalysts could potentially be screened and ranked in less than a day, and 3) accurate, and with a wide dynamic range, as demonstrated by the comparison to HPLC determinations from 100% ee (S)-2 to 100% ee (R)-2. However, since the method is based on the catalysis of a reference reaction, the intent is to provide a HTS to obtain a subset of catalysts that produce the highest ee values. Then, these candidates can be tested and rigorously analyzed, in this case, for efficiency in preparing other amino acids of interest. Importantly, bluefluorescent HTS has broad applicability. By using the preparation of the same reference compounds, catalysts of other types could be evaluated in both alkylation and nonalkylation strategies for amino acid syntheses. For example, the latter could include Jacobsen-type catalysts for the Strecker reaction.[20] Finally, we are also currently extending the diversity of the trans-stilbene moiety, which will allow the application of the blue-fluorescent mAb sensor in screening asymmetric catalysis for other CC bond-forming reactions, as well as other classes of reactions, such as oxidations, reductions, and hydrolyses. Received: September 4, 2003 [Z52793] Scheme 3. Synthesis of chiral ligands for mAb 19G2 by using Cinchona-based PTC. Figure 1. The ee values from Cinchona-based PTC measured with the mAb 19G2 sensor. Communications 5986 2003 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.angewandte.org Angew. Chem. Int. Ed. 2003, 42, 5984 –5987���