正在加载图片...
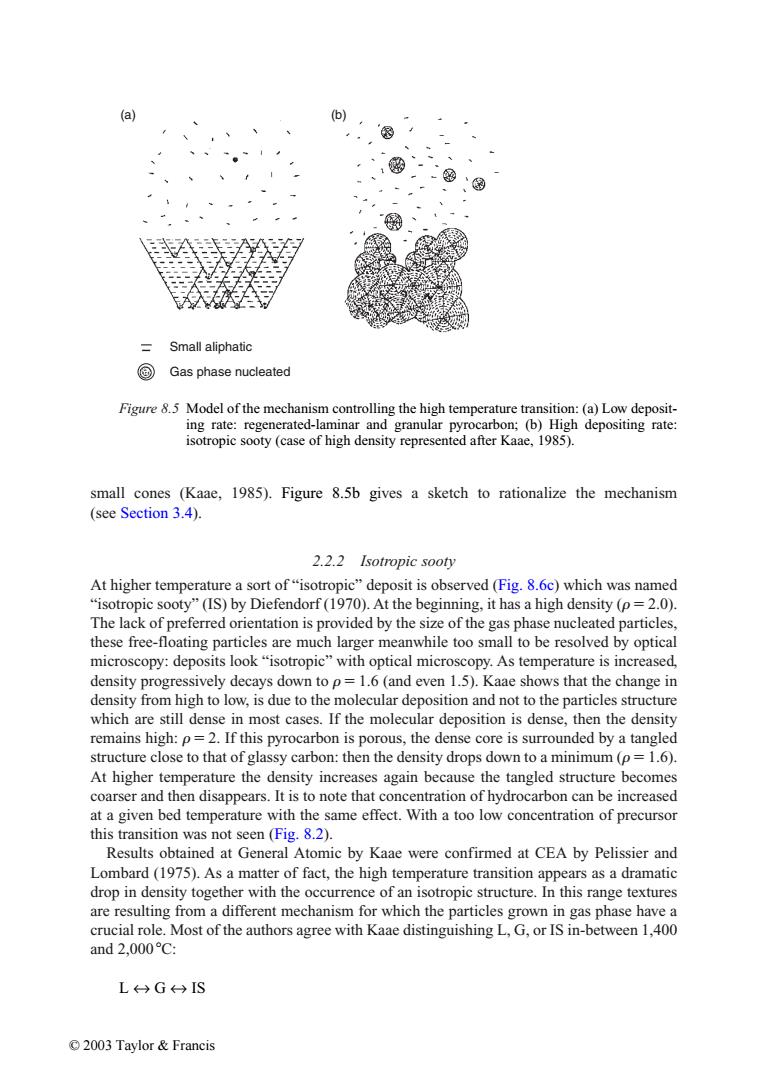
(a) b 三 Small aliphatic @ Gas phase nucleated Figure 8.5 Model of the mechanism controlling the high temperature transition:(a)Low deposit- ing rate:regenerated-laminar and granular pyrocarbon;(b)High depositing rate: isotropic sooty(case of high density represented after Kaae,1985). small cones (Kaae,1985).Figure 8.5b gives a sketch to rationalize the mechanism (see Section 3.4). 2.2.2 Isotropic sooty At higher temperature a sort of"isotropic"deposit is observed(Fig.8.6c)which was named "isotropic sooty"(IS)by Diefendorf(1970).At the beginning,it has a high density (p=2.0). The lack of preferred orientation is provided by the size of the gas phase nucleated particles, these free-floating particles are much larger meanwhile too small to be resolved by optical microscopy:deposits look"isotropic"with optical microscopy.As temperature is increased, density progressively decays down to p=1.6(and even 1.5).Kaae shows that the change in density from high to low,is due to the molecular deposition and not to the particles structure which are still dense in most cases.If the molecular deposition is dense,then the density remains high:p=2.If this pyrocarbon is porous,the dense core is surrounded by a tangled structure close to that of glassy carbon:then the density drops down to a minimum (p=1.6). At higher temperature the density increases again because the tangled structure becomes coarser and then disappears.It is to note that concentration of hydrocarbon can be increased at a given bed temperature with the same effect.With a too low concentration of precursor this transition was not seen (Fig.8.2). Results obtained at General Atomic by Kaae were confirmed at CEA by Pelissier and Lombard(1975).As a matter of fact,the high temperature transition appears as a dramatic drop in density together with the occurrence of an isotropic structure.In this range textures are resulting from a different mechanism for which the particles grown in gas phase have a crucial role.Most of the authors agree with Kaae distinguishing L,G,or IS in-between 1,400 and2,000c: L←→G←→IS ©2003 Taylor&FrancisFigure 8.5 Model of the mechanism controlling the high temperature transition: (a) Low depositing rate: regenerated-laminar and granular pyrocarbon; (b) High depositing rate: isotropic sooty (case of high density represented after Kaae, 1985). Gas phase nucleated Small aliphatic (a) (b) small cones (Kaae, 1985). Figure 8.5b gives a sketch to rationalize the mechanism (see Section 3.4). 2.2.2 Isotropic sooty At higher temperature a sort of “isotropic” deposit is observed (Fig. 8.6c) which was named “isotropic sooty” (IS) by Diefendorf (1970). At the beginning, it has a high density ( 2.0). The lack of preferred orientation is provided by the size of the gas phase nucleated particles, these free-floating particles are much larger meanwhile too small to be resolved by optical microscopy: deposits look “isotropic” with optical microscopy. As temperature is increased, density progressively decays down to 1.6 (and even 1.5). Kaae shows that the change in density from high to low, is due to the molecular deposition and not to the particles structure which are still dense in most cases. If the molecular deposition is dense, then the density remains high: 2. If this pyrocarbon is porous, the dense core is surrounded by a tangled structure close to that of glassy carbon: then the density drops down to a minimum ( 1.6). At higher temperature the density increases again because the tangled structure becomes coarser and then disappears. It is to note that concentration of hydrocarbon can be increased at a given bed temperature with the same effect. With a too low concentration of precursor this transition was not seen (Fig. 8.2). Results obtained at General Atomic by Kaae were confirmed at CEA by Pelissier and Lombard (1975). As a matter of fact, the high temperature transition appears as a dramatic drop in density together with the occurrence of an isotropic structure. In this range textures are resulting from a different mechanism for which the particles grown in gas phase have a crucial role. Most of the authors agree with Kaae distinguishing L, G, or IS in-between 1,400 and 2,000 C: L ↔ G ↔ IS © 2003 Taylor & Francis����