正在加载图片...
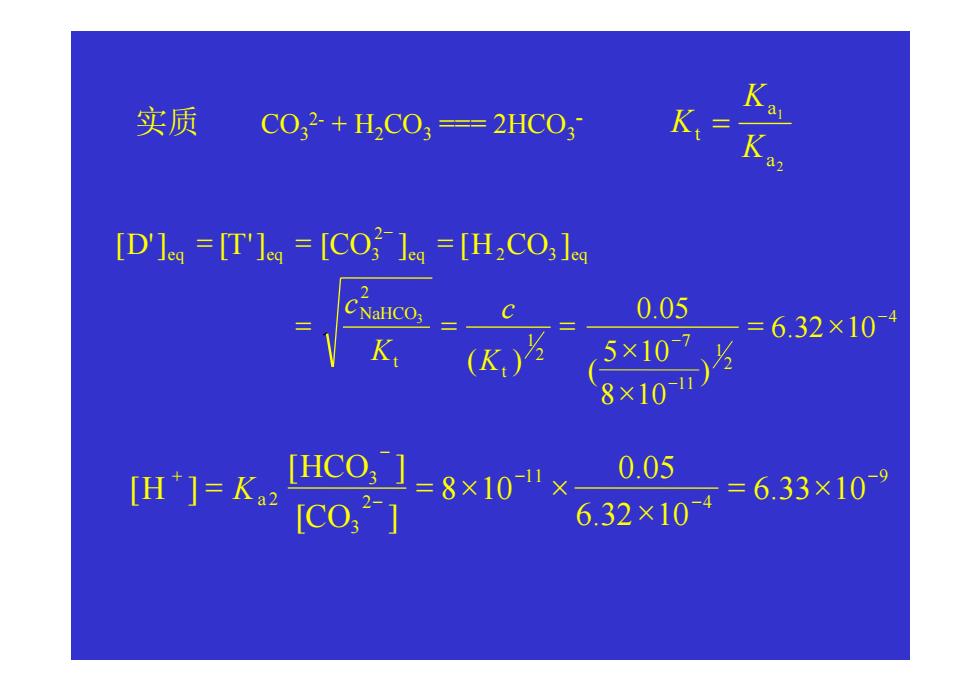
实质 CO32+HC03=2HC03 K [D'le=[T'lea=[COled =[H2CO;le 2 0.05 5×107 =632×104 K(K) 8707 m=kHC01=8x10"× 0.05 [co,*] 6,32×10=6,33×109CO32- + H2CO3 === 2HCO3 实质 - 2 1 a a t K K K = 4 2 1 11 7 2 1 2 2 3 2 3 6.32 10 ) 8 10 5 10 ( 0.05 ( ) [ '] [ '] [ ] [ ] 3 − − − − = × × × = = = = = = t t NaHCO eq eq eq eq K c K c D T CO H CO 9 4 11 2 3 3 2 6.33 10 6.32 10 0.05 8 10 [ ] [ ] [ ] − − − − − + = × × = = × × CO HCO H Ka