正在加载图片...
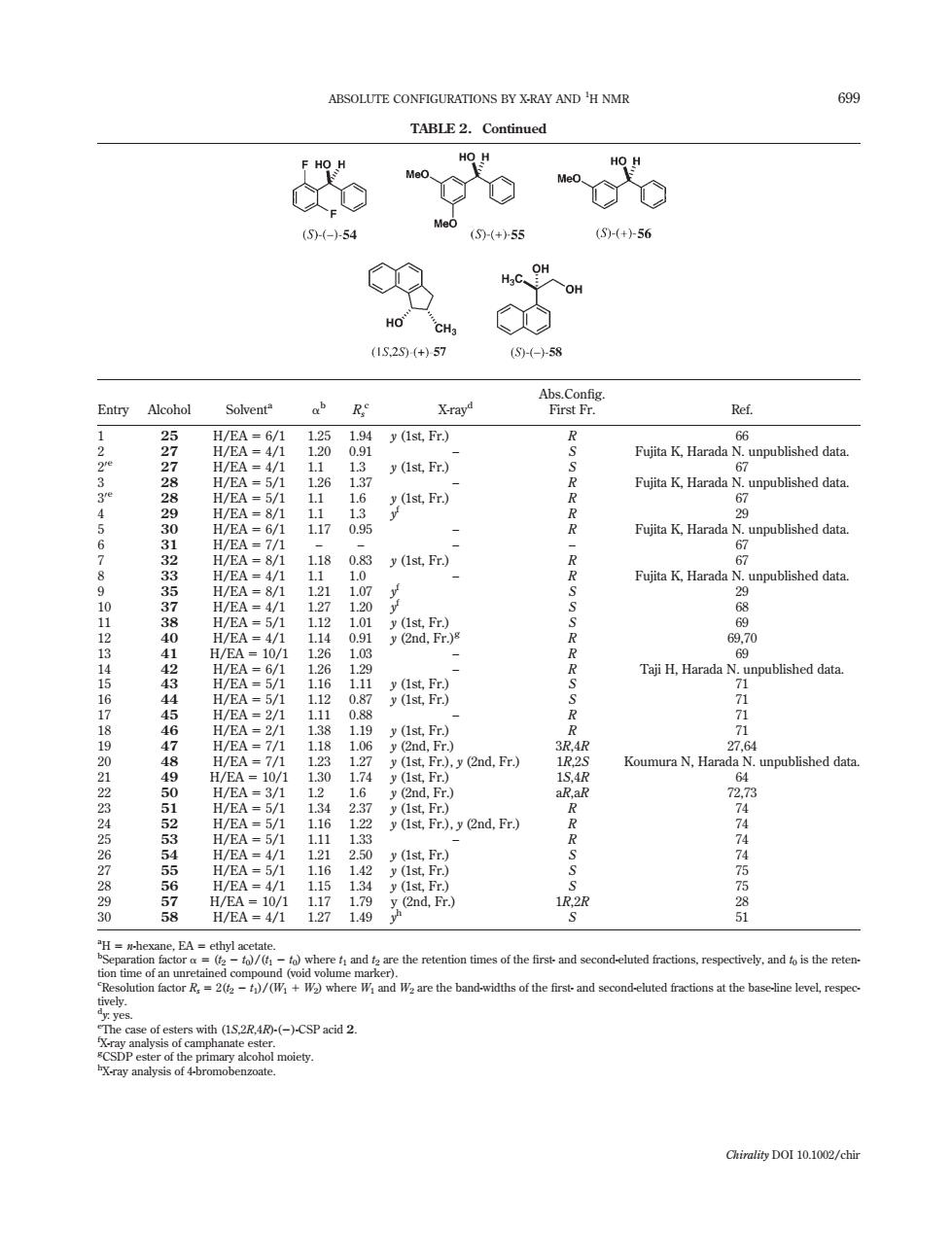
ABSOLUTE CONFIGURATIONS BY X-RAY AND 'H NMR 69g TABLE2.Continued HO H oCO (6(-)54 Me0(5)55 +-56 (15.25(+)57 59-58 Entry Alcoho Solvent ab X-ray SP FaKHarnbNiLnpubishedih -5/1 y(Ist.Fr.) 53 Fujita K.Harada N (lst.Fr) o 33456789012314151671890234567890 57776880900132853784411431441561118140012854558678 Fujita K.Harad y(Ist,Fr.) =5 n (n 69 TiH.Harada N.upublished data (2nd.Fr.) unpublished data 1R.2R 4444558 @where f:and 1 tion times o对hei ctions,respectively,and to is the reten t-and seo d-eluted the bas level respe ih()-()-CSPacid 2 y al moiety Chirality DOI10.12/chirTABLE 2. Continued Entry Alcohol Solventa ab Rs c X-rayd Abs.Config. First Fr. Ref. 1 25 H/EA 5 6/1 1.25 1.94 y (1st, Fr.) R 66 2 27 H/EA 5 4/1 1.20 0.91 – S Fujita K, Harada N. unpublished data. 20e 27 H/EA 5 4/1 1.1 1.3 y (1st, Fr.) S 67 3 28 H/EA 5 5/1 1.26 1.37 – R Fujita K, Harada N. unpublished data. 30e 28 H/EA 5 5/1 1.1 1.6 y (1st, Fr.) R 67 4 29 H/EA 5 8/1 1.1 1.3 y f R 29 5 30 H/EA 5 6/1 1.17 0.95 – R Fujita K, Harada N. unpublished data. 6 31 H/EA 5 7/1 – – – – 67 7 32 H/EA 5 8/1 1.18 0.83 y (1st, Fr.) R 67 8 33 H/EA 5 4/1 1.1 1.0 – R Fujita K, Harada N. unpublished data. 9 35 H/EA 5 8/1 1.21 1.07 y f S 29 10 37 H/EA 5 4/1 1.27 1.20 y f S 68 11 38 H/EA 5 5/1 1.12 1.01 y (1st, Fr.) S 69 12 40 H/EA 5 4/1 1.14 0.91 y (2nd, Fr.)g R 69,70 13 41 H/EA 5 10/1 1.26 1.03 – R 69 14 42 H/EA 5 6/1 1.26 1.29 – R Taji H, Harada N. unpublished data. 15 43 H/EA 5 5/1 1.16 1.11 y (1st, Fr.) S 71 16 44 H/EA 5 5/1 1.12 0.87 y (1st, Fr.) S 71 17 45 H/EA 5 2/1 1.11 0.88 – R 71 18 46 H/EA 5 2/1 1.38 1.19 y (1st, Fr.) R 71 19 47 H/EA 5 7/1 1.18 1.06 y (2nd, Fr.) 3R,4R 27,64 20 48 H/EA 5 7/1 1.23 1.27 y (1st, Fr.), y (2nd, Fr.) 1R,2S Koumura N, Harada N. unpublished data. 21 49 H/EA 5 10/1 1.30 1.74 y (1st, Fr.) 1S,4R 64 22 50 H/EA 5 3/1 1.2 1.6 y (2nd, Fr.) aR,aR 72,73 23 51 H/EA 5 5/1 1.34 2.37 y (1st, Fr.) R 74 24 52 H/EA 5 5/1 1.16 1.22 y (1st, Fr.), y (2nd, Fr.) R 74 25 53 H/EA 5 5/1 1.11 1.33 – R 74 26 54 H/EA 5 4/1 1.21 2.50 y (1st, Fr.) S 74 27 55 H/EA 5 5/1 1.16 1.42 y (1st, Fr.) S 75 28 56 H/EA 5 4/1 1.15 1.34 y (1st, Fr.) S 75 29 57 H/EA 5 10/1 1.17 1.79 y (2nd, Fr.) 1R,2R 28 30 58 H/EA 5 4/1 1.27 1.49 y h S 51 a H 5 n-hexane, EA 5 ethyl acetate. b Separation factor a 5 (t2 2 t0)/(t1 2 t0) where t1 and t2 are the retention times of the first- and second-eluted fractions, respectively, and t0 is the retention time of an unretained compound (void volume marker). c Resolution factor Rs 5 2(t2 2 t1)/(W1 1 W2) where W1 and W2 are the band-widths of the first- and second-eluted fractions at the base-line level, respectively. d y: yes. e The case of esters with (1S,2R,4R)-(2)-CSP acid 2. f X-ray analysis of camphanate ester. g CSDP ester of the primary alcohol moiety. h X-ray analysis of 4-bromobenzoate. ABSOLUTE CONFIGURATIONS BY X-RAY AND 699 1 H NMR Chirality DOI 10.1002/chir