正在加载图片...
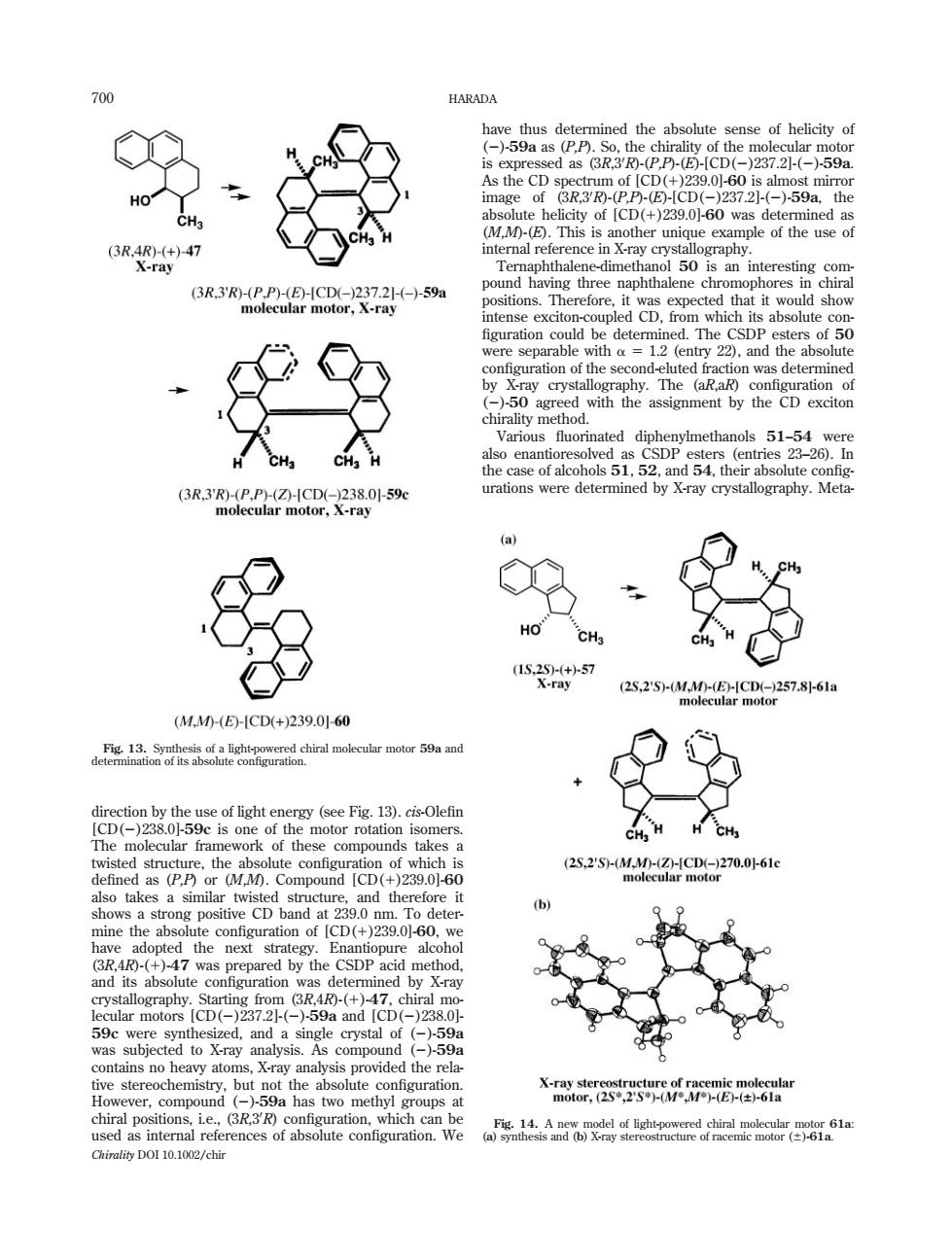
HARADA s-CD(27)50 6R7 figuration could be dete absol hirality method. se of alcohols 51. 52.and 54.the X-ray crystallography s2SMME9e257s6a (M.M0-(E-CD(+)239.060 ework of these compounds t 2s2S9-MMCDe270.061 -()7was ne y X-ray e stereochems59a has two methygo the e mtor ad recr c Chirality DOI 10.1002/chir direction by the use of light energy (see Fig. 13). cis-Olefin [CD(2)238.0]-59c is one of the motor rotation isomers. The molecular framework of these compounds takes a twisted structure, the absolute configuration of which is defined as (P,P) or (M,M). Compound [CD(1)239.0]-60 also takes a similar twisted structure, and therefore it shows a strong positive CD band at 239.0 nm. To determine the absolute configuration of [CD(1)239.0]-60, we have adopted the next strategy. Enantiopure alcohol (3R,4R)-(1)-47 was prepared by the CSDP acid method, and its absolute configuration was determined by X-ray crystallography. Starting from (3R,4R)-(1)-47, chiral molecular motors [CD(2)237.2]-(2)-59a and [CD(2)238.0]- 59c were synthesized, and a single crystal of (2)-59a was subjected to X-ray analysis. As compound (2)-59a contains no heavy atoms, X-ray analysis provided the relative stereochemistry, but not the absolute configuration. However, compound (2)-59a has two methyl groups at chiral positions, i.e., (3R,30 R) configuration, which can be used as internal references of absolute configuration. We have thus determined the absolute sense of helicity of (2)-59a as (P,P). So, the chirality of the molecular motor is expressed as (3R,30 R)-(P,P)-(E)-[CD(2)237.2]-(2)-59a. As the CD spectrum of [CD(1)239.0]-60 is almost mirror image of (3R,30 R)-(P,P)-(E)-[CD(2)237.2]-(2)-59a, the absolute helicity of [CD(1)239.0]-60 was determined as (M,M)-(E). This is another unique example of the use of internal reference in X-ray crystallography. Ternaphthalene-dimethanol 50 is an interesting compound having three naphthalene chromophores in chiral positions. Therefore, it was expected that it would show intense exciton-coupled CD, from which its absolute con- figuration could be determined. The CSDP esters of 50 were separable with a 5 1.2 (entry 22), and the absolute configuration of the second-eluted fraction was determined by X-ray crystallography. The (aR,aR) configuration of (2)-50 agreed with the assignment by the CD exciton chirality method. Various fluorinated diphenylmethanols 51–54 were also enantioresolved as CSDP esters (entries 23–26). In the case of alcohols 51, 52, and 54, their absolute configurations were determined by X-ray crystallography. MetaFig. 13. Synthesis of a light-powered chiral molecular motor 59a and determination of its absolute configuration. Fig. 14. A new model of light-powered chiral molecular motor 61a: (a) synthesis and (b) X-ray stereostructure of racemic motor (6)-61a. 700 HARADA Chirality DOI 10.1002/chir