正在加载图片...
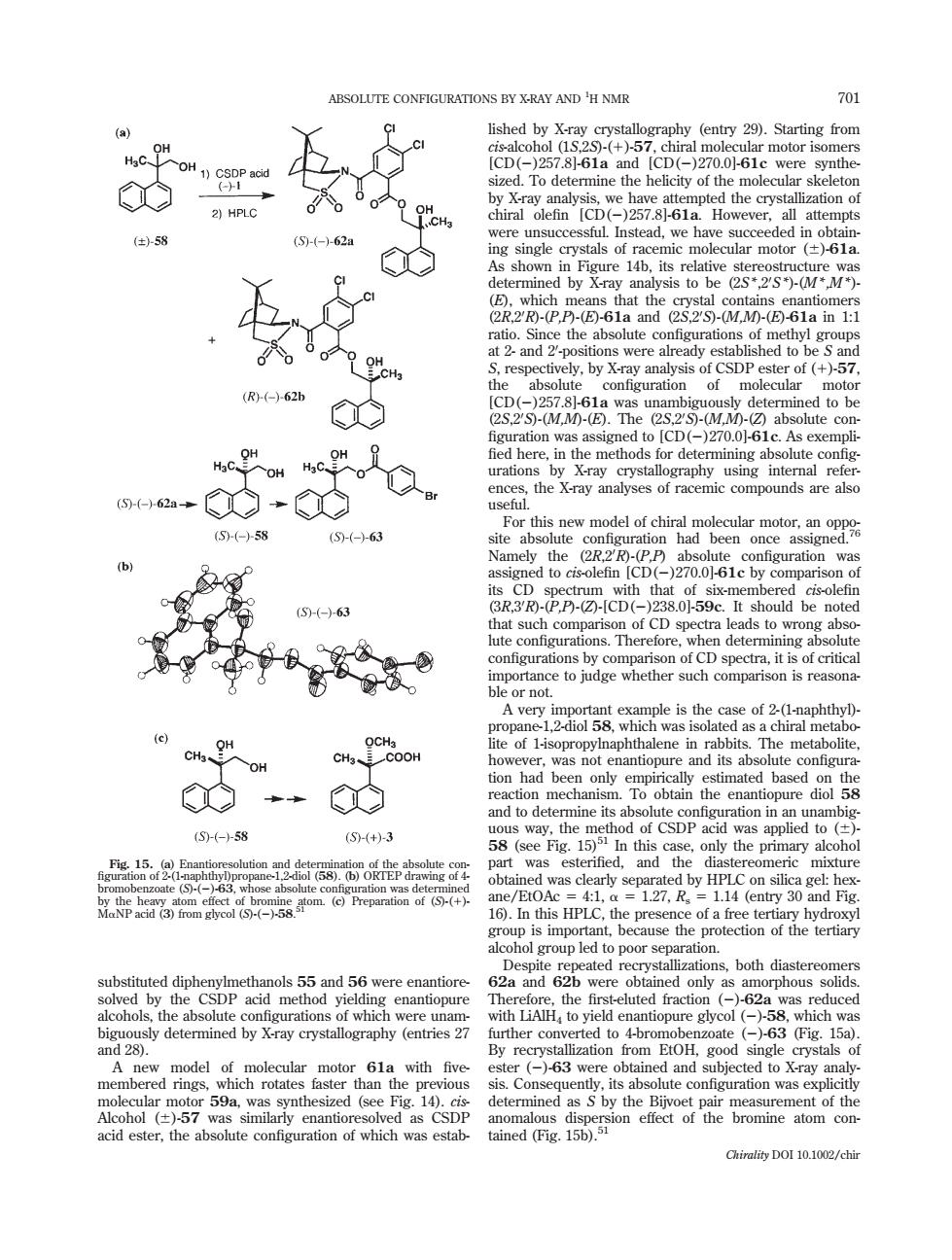
ABSOLUTE CONFIGURATIONS BY X-RAY AND 'H NMR dgX5o邮emiy2.Saring rom 78161 ize 2)HPLC ( 人。士分 the R)-(-)62 rations graphy using internal refe the Xray analyses of racen mic compounds are alse 5621 ⑤-(58 configuration was with that of si r not cH、gH OH had ⑤(-58 (6()3 58 picdnamb the by HPLC on lc mixture is HPLO 127,R 14 (entry 30 and Fig ol group led to poors ns.both diaste eitedpbepettok.55and56wereemantore 2a and 62b were only as orphous with LiAH to yield en acid ester,the Chirality DOI 10.1002/chirsubstituted diphenylmethanols 55 and 56 were enantioresolved by the CSDP acid method yielding enantiopure alcohols, the absolute configurations of which were unambiguously determined by X-ray crystallography (entries 27 and 28). A new model of molecular motor 61a with fivemembered rings, which rotates faster than the previous molecular motor 59a, was synthesized (see Fig. 14). cisAlcohol (6)-57 was similarly enantioresolved as CSDP acid ester, the absolute configuration of which was established by X-ray crystallography (entry 29). Starting from cis-alcohol (1S,2S)-(1)-57, chiral molecular motor isomers [CD(2)257.8]-61a and [CD(2)270.0]-61c were synthesized. To determine the helicity of the molecular skeleton by X-ray analysis, we have attempted the crystallization of chiral olefin [CD(2)257.8]-61a. However, all attempts were unsuccessful. Instead, we have succeeded in obtaining single crystals of racemic molecular motor (6)-61a. As shown in Figure 14b, its relative stereostructure was determined by X-ray analysis to be (2S*,20 S*)-(M*,M*)- (E), which means that the crystal contains enantiomers (2R,20 R)-(P,P)-(E)-61a and (2S,20 S)-(M,M)-(E)-61a in 1:1 ratio. Since the absolute configurations of methyl groups at 2- and 20 -positions were already established to be S and S, respectively, by X-ray analysis of CSDP ester of (1)-57, the absolute configuration of molecular motor [CD(2)257.8]-61a was unambiguously determined to be (2S,20 S)-(M,M)-(E). The (2S,20 S)-(M,M)-(Z) absolute con- figuration was assigned to [CD(2)270.0]-61c. As exempli- fied here, in the methods for determining absolute configurations by X-ray crystallography using internal references, the X-ray analyses of racemic compounds are also useful. For this new model of chiral molecular motor, an opposite absolute configuration had been once assigned.76 Namely the (2R,20 R)-(P,P) absolute configuration was assigned to cis-olefin [CD(2)270.0]-61c by comparison of its CD spectrum with that of six-membered cis-olefin (3R,30 R)-(P,P)-(Z)-[CD(2)238.0]-59c. It should be noted that such comparison of CD spectra leads to wrong absolute configurations. Therefore, when determining absolute configurations by comparison of CD spectra, it is of critical importance to judge whether such comparison is reasonable or not. A very important example is the case of 2-(1-naphthyl)- propane-1,2-diol 58, which was isolated as a chiral metabolite of 1-isopropylnaphthalene in rabbits. The metabolite, however, was not enantiopure and its absolute configuration had been only empirically estimated based on the reaction mechanism. To obtain the enantiopure diol 58 and to determine its absolute configuration in an unambiguous way, the method of CSDP acid was applied to (6)- 58 (see Fig. 15)51 In this case, only the primary alcohol part was esterified, and the diastereomeric mixture obtained was clearly separated by HPLC on silica gel: hexane/EtOAc 5 4:1, a 5 1.27, Rs 5 1.14 (entry 30 and Fig. 16). In this HPLC, the presence of a free tertiary hydroxyl group is important, because the protection of the tertiary alcohol group led to poor separation. Despite repeated recrystallizations, both diastereomers 62a and 62b were obtained only as amorphous solids. Therefore, the first-eluted fraction (2)-62a was reduced with LiAlH4 to yield enantiopure glycol (2)-58, which was further converted to 4-bromobenzoate (2)-63 (Fig. 15a). By recrystallization from EtOH, good single crystals of ester (2)-63 were obtained and subjected to X-ray analysis. Consequently, its absolute configuration was explicitly determined as S by the Bijvoet pair measurement of the anomalous dispersion effect of the bromine atom contained (Fig. 15b).51 Fig. 15. (a) Enantioresolution and determination of the absolute con- figuration of 2-(1-naphthyl)propane-1,2-diol (58). (b) ORTEP drawing of 4- bromobenzoate (S)-(2)-63, whose absolute configuration was determined by the heavy atom effect of bromine atom. (c) Preparation of (S)-(1)- MaNP acid (3) from glycol (S)-(2)-58. 51 ABSOLUTE CONFIGURATIONS BY X-RAY AND 701 1 H NMR Chirality DOI 10.1002/chir