正在加载图片...
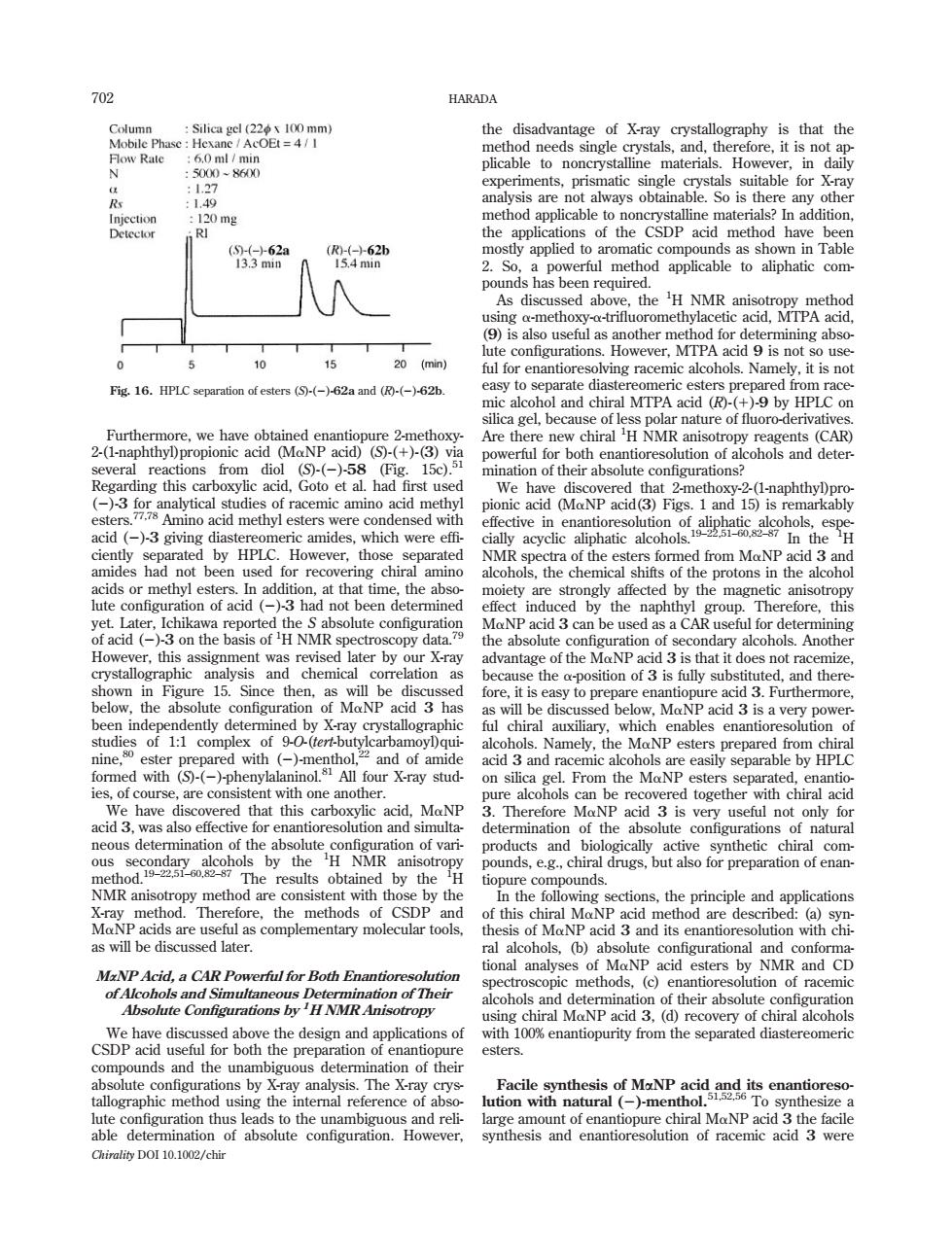
702 HARADA 0 mg R台62n as shown in Tabl 5 20 (min) .HPLC separation of esters (5(-)-62a and (-)-62b. min acid ()3 giving 地w acid ( an chemical which ena ine. siste nt y with one anothe prepa The the methods CSDE ons of this NP acid 3 and its enantioresolution wi of MaNP acid esters by NMR and CD MaNP Acid,a CAR Powerful for Both Enantioresolution 3,(④reco esters mbiguou enantio l usin the Chirality DOI 10.1002/chir Furthermore, we have obtained enantiopure 2-methoxy- 2-(1-naphthyl)propionic acid (MaNP acid) (S)-(1)-(3) via several reactions from diol (S)-(2)-58 (Fig. 15c).51 Regarding this carboxylic acid, Goto et al. had first used (2)-3 for analytical studies of racemic amino acid methyl esters.77,78 Amino acid methyl esters were condensed with acid (2)-3 giving diastereomeric amides, which were effi- ciently separated by HPLC. However, those separated amides had not been used for recovering chiral amino acids or methyl esters. In addition, at that time, the absolute configuration of acid (2)-3 had not been determined yet. Later, Ichikawa reported the S absolute configuration of acid (2)-3 on the basis of 1 H NMR spectroscopy data.79 However, this assignment was revised later by our X-ray crystallographic analysis and chemical correlation as shown in Figure 15. Since then, as will be discussed below, the absolute configuration of MaNP acid 3 has been independently determined by X-ray crystallographic studies of 1:1 complex of 9-O-(tert-butylcarbamoyl)quinine,80 ester prepared with (2)-menthol,22 and of amide formed with (S)-(2)-phenylalaninol.81 All four X-ray studies, of course, are consistent with one another. We have discovered that this carboxylic acid, MaNP acid 3, was also effective for enantioresolution and simultaneous determination of the absolute configuration of various secondary alcohols by the 1 H NMR anisotropy method.19–22,51–60,82–87 The results obtained by the 1 H NMR anisotropy method are consistent with those by the X-ray method. Therefore, the methods of CSDP and MaNP acids are useful as complementary molecular tools, as will be discussed later. MaNP Acid, a CAR Powerful for Both Enantioresolution of Alcohols and Simultaneous Determination of Their Absolute Configurations by 1H NMR Anisotropy We have discussed above the design and applications of CSDP acid useful for both the preparation of enantiopure compounds and the unambiguous determination of their absolute configurations by X-ray analysis. The X-ray crystallographic method using the internal reference of absolute configuration thus leads to the unambiguous and reliable determination of absolute configuration. However, the disadvantage of X-ray crystallography is that the method needs single crystals, and, therefore, it is not applicable to noncrystalline materials. However, in daily experiments, prismatic single crystals suitable for X-ray analysis are not always obtainable. So is there any other method applicable to noncrystalline materials? In addition, the applications of the CSDP acid method have been mostly applied to aromatic compounds as shown in Table 2. So, a powerful method applicable to aliphatic compounds has been required. As discussed above, the 1 H NMR anisotropy method using a-methoxy-a-trifluoromethylacetic acid, MTPA acid, (9) is also useful as another method for determining absolute configurations. However, MTPA acid 9 is not so useful for enantioresolving racemic alcohols. Namely, it is not easy to separate diastereomeric esters prepared from racemic alcohol and chiral MTPA acid (R)-(1)-9 by HPLC on silica gel, because of less polar nature of fluoro-derivatives. Are there new chiral 1 H NMR anisotropy reagents (CAR) powerful for both enantioresolution of alcohols and determination of their absolute configurations? We have discovered that 2-methoxy-2-(1-naphthyl)propionic acid (MaNP acid(3) Figs. 1 and 15) is remarkably effective in enantioresolution of aliphatic alcohols, especially acyclic aliphatic alcohols.19–22,51–60,82–87 In the 1 H NMR spectra of the esters formed from MaNP acid 3 and alcohols, the chemical shifts of the protons in the alcohol moiety are strongly affected by the magnetic anisotropy effect induced by the naphthyl group. Therefore, this MaNP acid 3 can be used as a CAR useful for determining the absolute configuration of secondary alcohols. Another advantage of the MaNP acid 3 is that it does not racemize, because the a-position of 3 is fully substituted, and therefore, it is easy to prepare enantiopure acid 3. Furthermore, as will be discussed below, MaNP acid 3 is a very powerful chiral auxiliary, which enables enantioresolution of alcohols. Namely, the MaNP esters prepared from chiral acid 3 and racemic alcohols are easily separable by HPLC on silica gel. From the MaNP esters separated, enantiopure alcohols can be recovered together with chiral acid 3. Therefore MaNP acid 3 is very useful not only for determination of the absolute configurations of natural products and biologically active synthetic chiral compounds, e.g., chiral drugs, but also for preparation of enantiopure compounds. In the following sections, the principle and applications of this chiral MaNP acid method are described: (a) synthesis of MaNP acid 3 and its enantioresolution with chiral alcohols, (b) absolute configurational and conformational analyses of MaNP acid esters by NMR and CD spectroscopic methods, (c) enantioresolution of racemic alcohols and determination of their absolute configuration using chiral MaNP acid 3, (d) recovery of chiral alcohols with 100% enantiopurity from the separated diastereomeric esters. Facile synthesis of MaNP acid and its enantioresolution with natural (2)-menthol.51,52,56 To synthesize a large amount of enantiopure chiral MaNP acid 3 the facile synthesis and enantioresolution of racemic acid 3 were Fig. 16. HPLC separation of esters (S)-(2)-62a and (R)-(2)-62b. 702 HARADA Chirality DOI 10.1002/chir