正在加载图片...
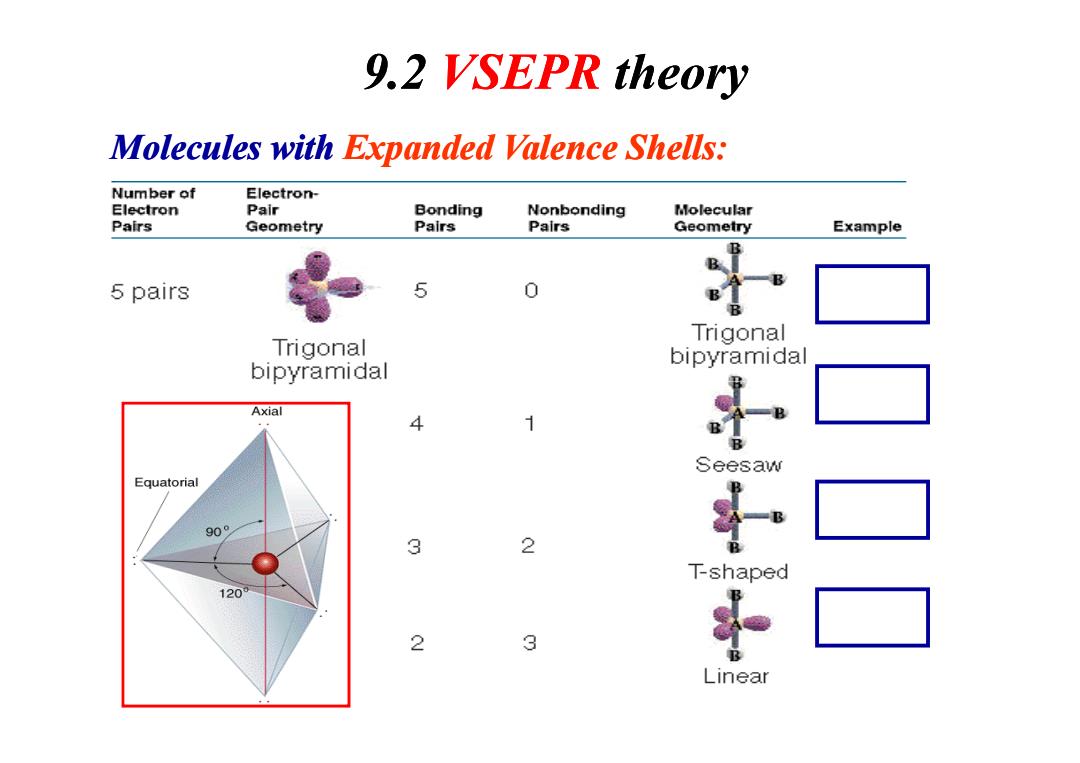
9.2 VSEPR theory Molecules with Expanded Valence Shells: Number of Electron- Electron Pair Bonding Nonbonding Molecular Pairs Geometry Pairs Pairs Geometry Example B 5 pairs 5 0 B Trigonal Trigonal bipyramidal bipyramidal Axial 4 1 B B Seesaw Equatorial 90° 3 2 T-shaped 120 2 3 LinearMolecules with Expanded Valence Shells: 9.2 VSEPR theory