Chap.9 Molecular Geometry and Bonding Theories 9.I Molecular Geometry 9.2 YSEPR model 9.3 Molecular Shape and Molecular Polarity 9.4 Covalent Bonding and Orbital Overlap 9.5 Hybridization Hybrid Orbitals 9.6 Multiple bond 9.7 Molecular Orbitals
Chap. 9 Molecular Geometry and Bonding Theories 9.1 Molecular Geometry 9.2 VSEPR model 9.3 Molecular Shape and Molecular Polarity 9.4 Covalent Bonding and Orbital Overlap 9.5 Hybridization & Hybrid 9.5 Hybridization & Hybrid Orbitals Orbitals 9.6 Multiple bond 9.7 Molecular 9.7 Molecular Orbitals Orbitals

Do SO2 CO2 and NO2 have the similar 3D structures? How about their Polarity?
Do SO2, CO2 and NO2 have the similar 3D structures? How about their Polarity?

Molecular Geometry (Shapes) Lewis structures give atomic connectivity 妇 The shape of a molecule is determined by its H&C&H bond angles OX H Bond distance, 1.78A Bond angle, 109.5
Molecular Geometry (Shapes) Lewis structures give atomic connectivity The shape of a molecule is determined by its bond angles H H H C Hx x x x
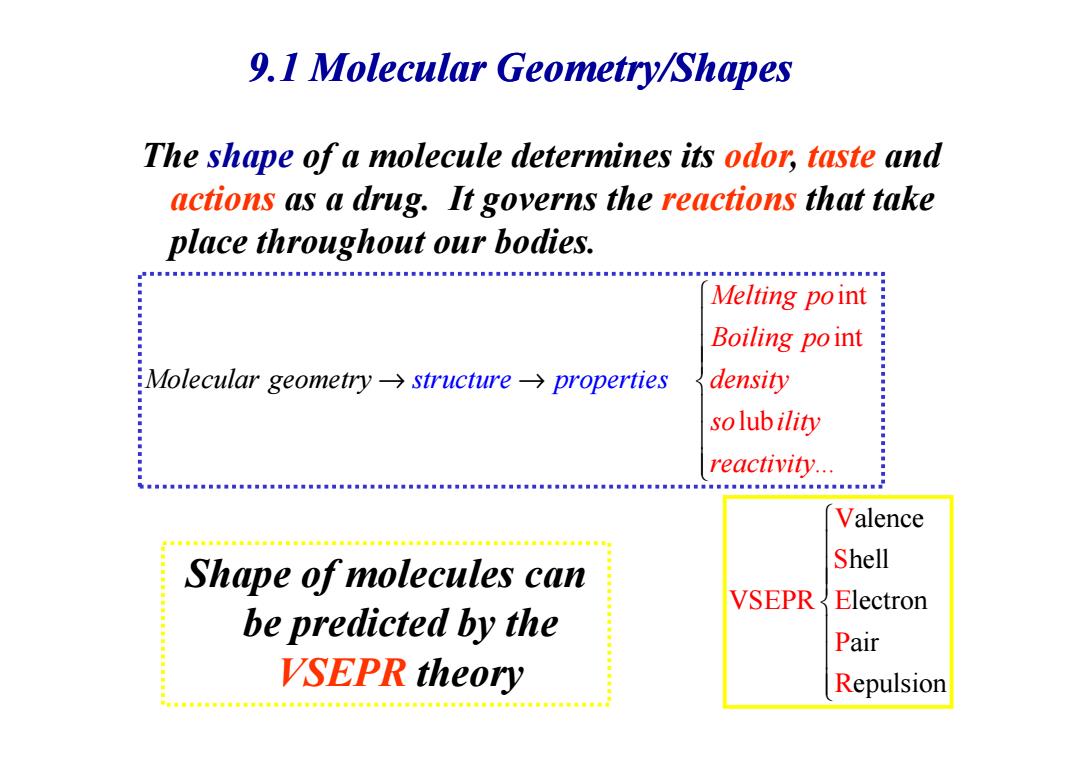
9.1 Molecular Geometry/Shapes The shape of a molecule determines its odor,taste and actions as a drug.It governs the reactions that take place throughout our bodies. Melting point Boiling point Molecular geometry->structure->properties density · solubility reactivity... Valence Shape of molecules can Shell VSEPR Electron be predicted by the Pair VSEPR theory Repulsion
9.1 Molecular Geometry/Shapes The shape of a molecule determines its odor, taste and actions as a drug. It governs the reactions that take place throughout our bodies. int int Melting po B Molecular geo oiling po metry structure properties density → → Shape of molecules can be predicted by the VSEPR theory lub ... Molecular geo density so ility structure reactivit metr prope y y → → rties alence hell lectron air epulsion V S VSEPR E P R

9.2 Valence Shell Electron Pair Repulsion (VSEPR)theory imt0predicttheshapesofaoleocule2 a)The valence electrons repel each other Bonding electrons Valence electrons of Central Atom- Non-bonding electrons b)The molecule adopts whichever 3D geometry minimized this repulsion. Valence electrons staying as far apart as possible!
9.2 Valence Shell Electron Pair Repulsion (VSEPR) theory a) The valence electrons repel each other - Bonding electro Valence ele ns Non bon ctrons din of Ce g elec nt tr r o al Atom ns b) The molecule adopts whichever 3D geometry minimized this repulsion. Valence electrons staying as far apart as possible!
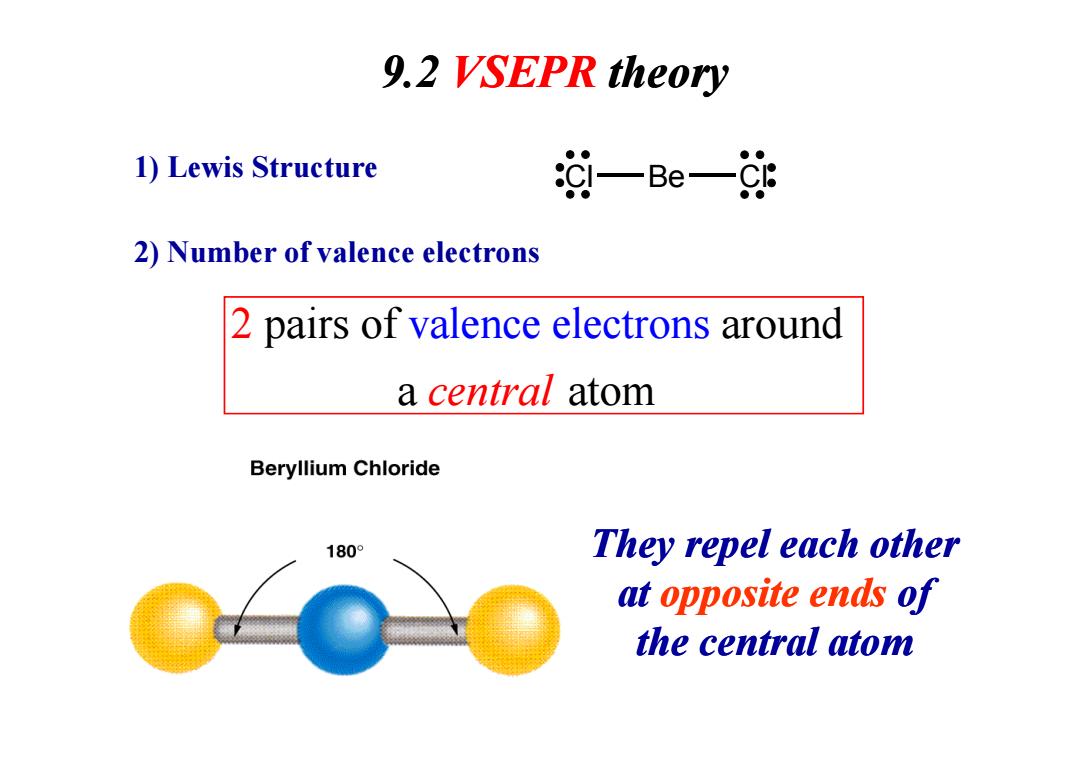
9.2 VSEPR theory 1)Lewis Structure 汝一Be一处 2)Number of valence electrons 2 pairs of valence electrons around a central atom Beryllium Chloride 180° They repel each other at opposite ends of the central atom
9.2 VSEPR theory pairs of around val a 2 ence ele a ctron t s central om 1) Lewis Structure Cl Be Cl 2) Number of valence electrons a central atom They repel each other at opposite ends of the central atom
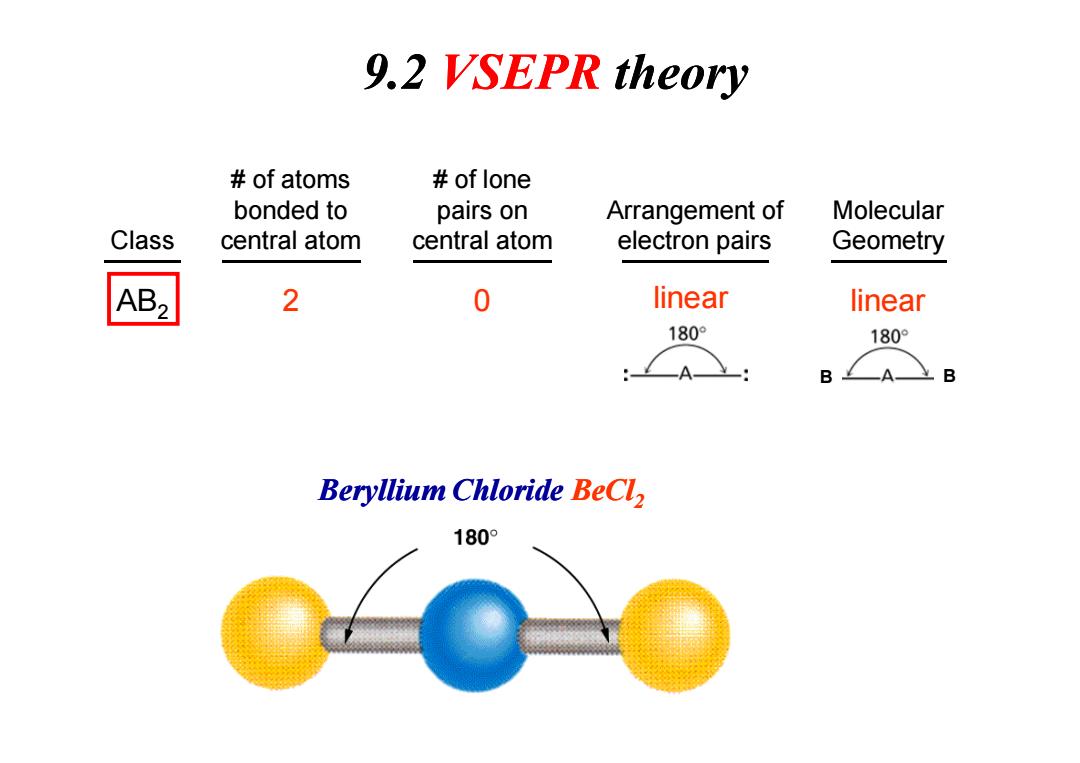
9.2 VSEPR theory of atoms of lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB2 2 0 linear linear 180° 180° :A Beryllium Chloride BeCl 180°
AB2 2 0 Class # of atoms bonded to central atom # of lone pairs on central atom Arrangement of electron pairs Molecular Geometry linear linear B B 9.2 VSEPR theory B B Beryllium Chloride BeCl2

9.2 VSEPR theory of atoms of lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB3 3 0 trigonal trigonal planar planar Boron Trifluoride BF3 120° \ Planar
Class # of atoms bonded to central atom # of lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB3 3 0 trigonal planar trigonal planar Boron Trifluoride BF3 9.2 VSEPR theory
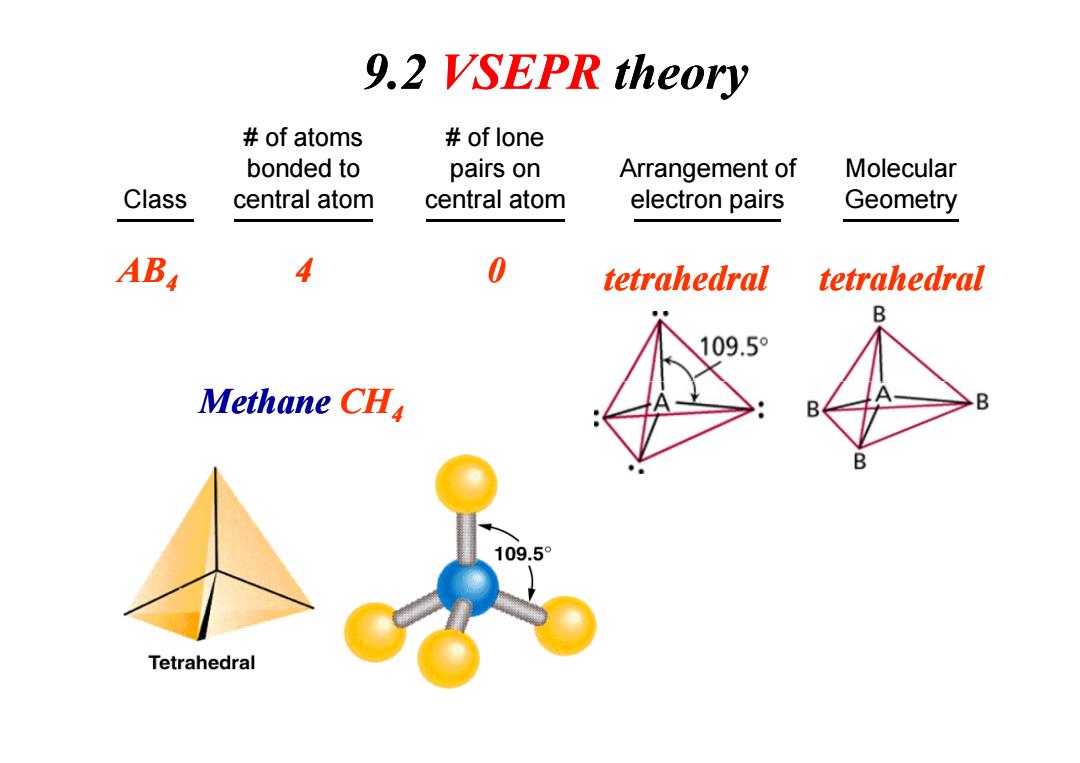
9.2 VSEPR theory of atoms of lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB 4 0 tetrahedral tetrahedral B 109.5° Methane CHa B B 109.5° Tetrahedral
Class # of atoms bonded to central atom # of lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB4 4 0 tetrahedral tetrahedral Methane CH 9.2 VSEPR theory Methane CH4
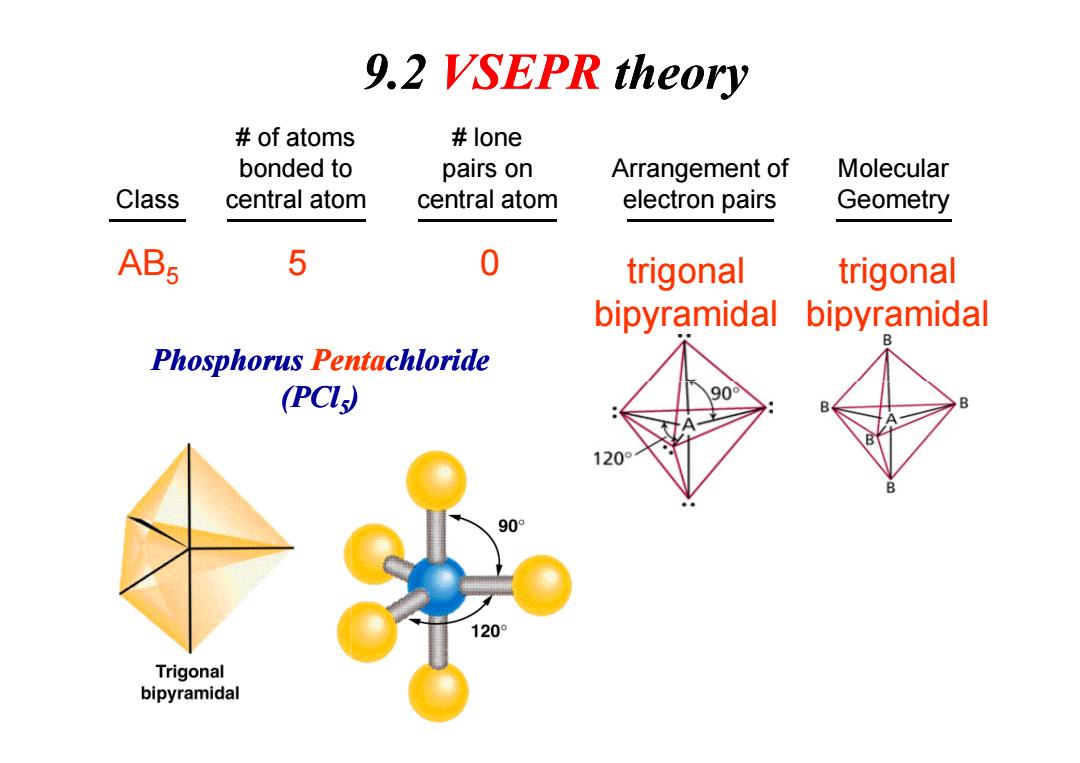
9.2 VSEPR theory of atoms lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB5 5 0 trigonal trigonal bipyramidal bipyramidal Phosphorus Pentachloride (PCL) 90 B 90° 120° Trigonal bipyramidal
Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB5 5 0 trigonal bipyramidal trigonal bipyramidal Phosphorus Pentachloride (PCl ) 9.2 VSEPR theory (PCl5)