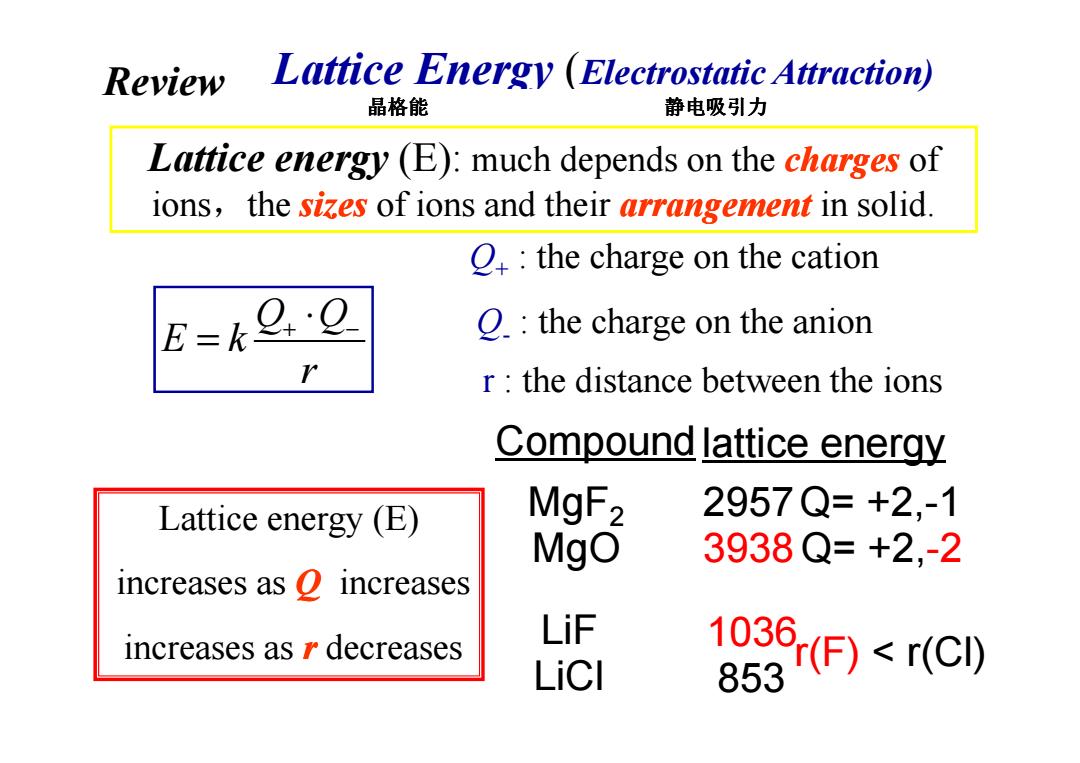
Review Lattice Energy (Electrostatic Attraction) 晶格能 静电吸引力 Lattice energy (E):much depends on the charges of ions,the sizes of ions and their arrangement in solid. O the charge on the cation E=k O.the charge on the anion r the distance between the ions Compound lattice energy Lattice energy (E) MgF2 2957Q=+2,-1 MgO 3938Q=+2,-2 increases as o increases LiF increases as r decreases LiCI (F)r(C) 1036
Lattice Energy (Electrostatic Attraction) Q+ : the charge on the cation Q- : the charge on the anion r : the distance between the ions Lattice energy (E): much depends on the charges of ions,the sizes of ions and their arrangement in solid. Q Q E k r + − ⋅ = 晶格能 静电吸引力 Review Lattice energy (E) increases as Q increases increases as r decreases lattice energy MgF2 MgO LiF LiCl 2957 3938 1036 853 Q= +2,-1 Q= +2,-2 r(F) < r(Cl) r : the distance between the ions Compound r
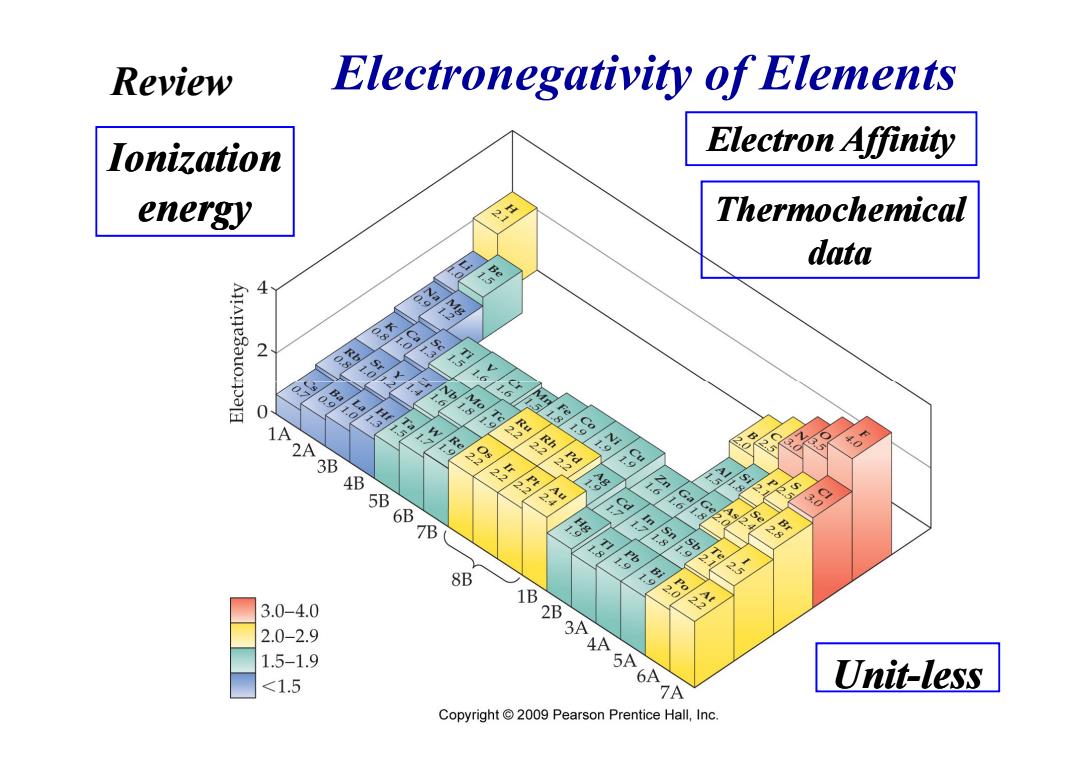
Review Electronegativity of Elements Ionization Electron Affinity energy Thermochemical data 系 39 0 88 的容 8 3B 68 1.5 8 59 e 9 6带 0 9 的平 8 8B 一 3.0-4.0 已之 2.0-2.9 1.5-1.9 5 <1.5 61P Unit-less Copyright 2009 Pearson Prentice Hall,Inc
Figure 08.06 Electronegativity of Elements Electron Affinity Ionization energy Thermochemical data Review Unit-less
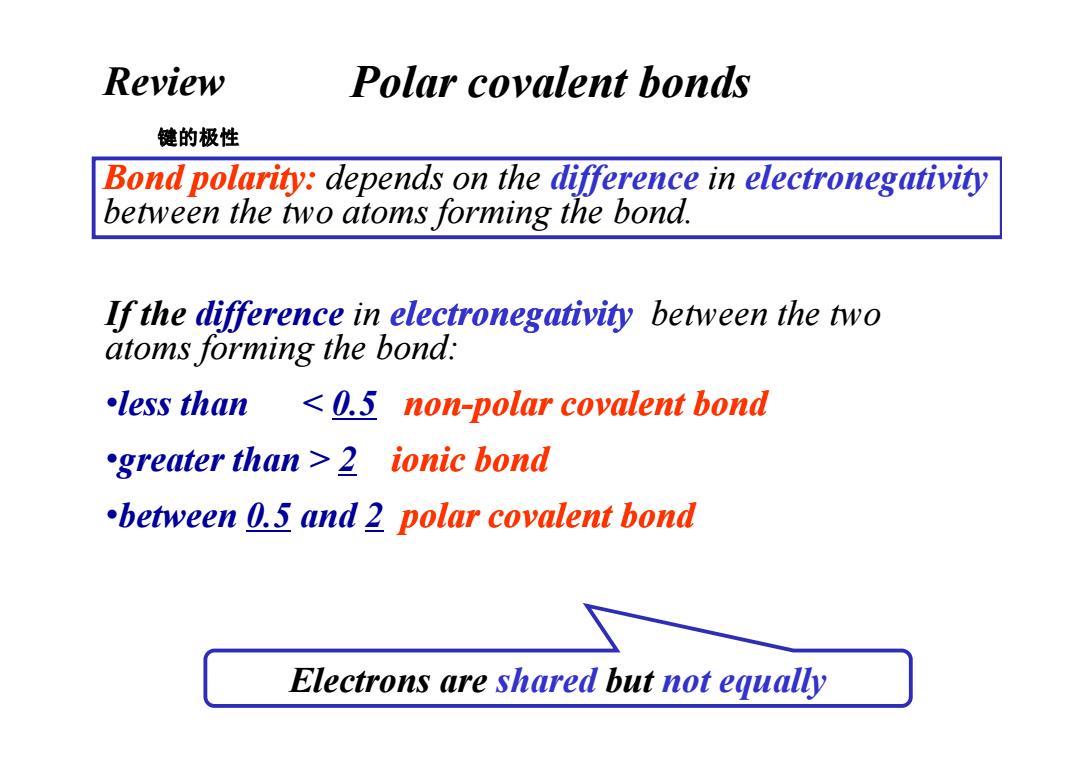
Review Polar covalent bonds 键的极性 Bond polarity:depends on the difference in electronegativity between the two atoms forming the bond. If the difference in electronegativity between the two atoms forming the bond: less than 0.5 non-polar covalent bond greater than 2 ionic bond between 0.5 and 2 polar covalent bond Electrons are shared but not equally
Polar covalent bonds Bond polarity: depends on the difference in electronegativity between the two atoms forming the bond. If the difference in electronegativity between the two atoms forming the bond: 键的极性 Review •less than 2 ionic bond •between 0.5 and 2 polar covalent bond Electrons are shared but not equally
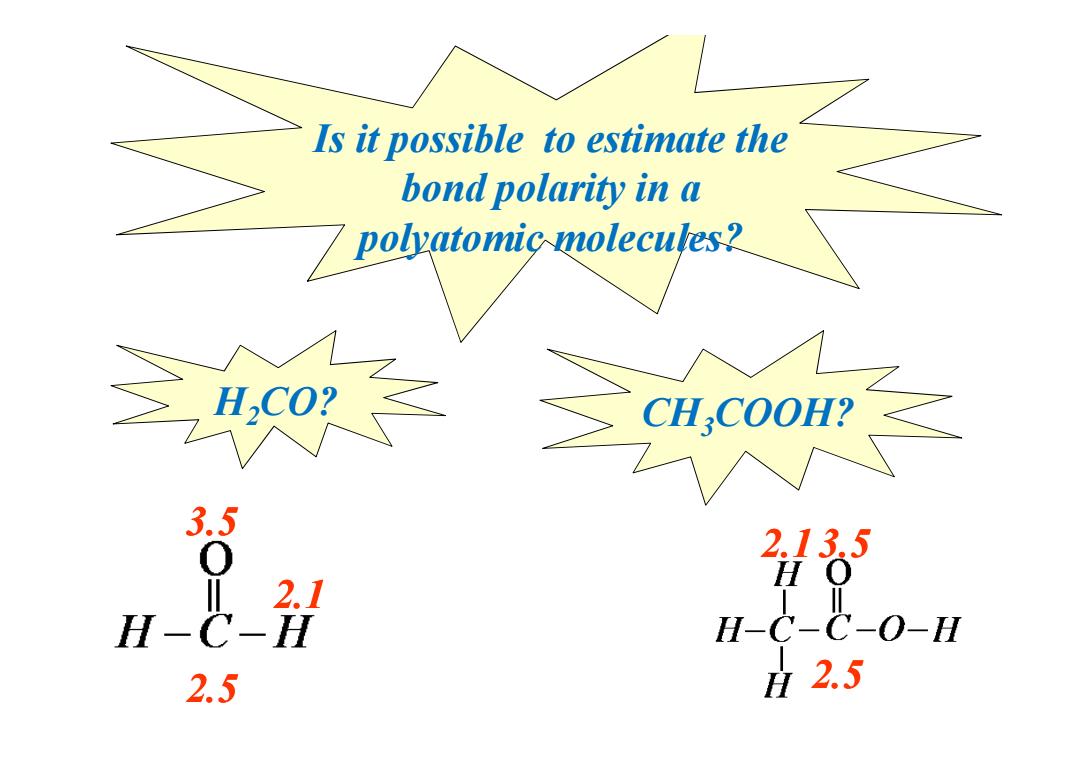
Is it possible to estimate the bond polarity in a 7 polyatomic molecules? CHCOOH? 3.5 2.135 心H H- H-C-C-O-H 2.5 2.5
Is it possible to estimate the bond polarity in a polyatomic molecules? H2CO? CH3COOH? 2.1 3.5 2.5 3.5 2.5 2.1

Solid at R.T; Lattice Hard and brittle; Energy: Conductivity? +788kJ/mol NaCl Strength of ionic bonds E=k alibaba.com.cn
Solid at R. T.; Hard and brittle; Conductivity? Lattice Energy: +788kJ/mol NaCl Q Q E k r + − ⋅ = Strength of ionic bonds E k r
Chap.9 Molecular Geometry and Bonding Theories 9.I Molecular Geometry 9.2 YSEPR model 9.3 Molecular Shape and Molecular Polarity 9.4 Covalent Bonding and Orbital Overlap 9.5 Hybridization Hybrid Orbitals 9.6 Multiple bond 9.7 Molecular Orbitals
Chap. 9 Molecular Geometry and Bonding Theories 9.1 Molecular Geometry 9.2 VSEPR model 9.3 Molecular Shape and Molecular Polarity 9.4 Covalent Bonding and Orbital Overlap 9.5 Hybridization & Hybrid Orbitals 9.6 Multiple bond 9.7 Molecular Orbitals

9.1 Molecular Geometry/Shape 分子构型 The shape of a molecule determines its odor,taste and actions as a drug.It governs the reactions that take place throughout our bodies. Melting po int 熔点 结构 性质 Boiling po int 沸点 :olecular geometry→structure→properties density 密度 solubility 溶解度 reactivity... 反应性 alibaba.com.cn crsce
9.1 Molecular Geometry/Shape The shape of a molecule determines its odor, taste and actions as a drug. It governs the reactions that take place throughout our bodies. int int Melting po B Molecular geo oiling po metry structure properties density → → 分子构型 熔点 沸点 密度 结构 性质 lub ... Molecular geo density so ility structure reactivit metr prope y y rties → → 密度 溶解度 反应性

Do SO2,CO2 and NO2 have the similar 3D structures? Polarity! 极性 Polar? Non-polar? 极性 非极性
Polar? Non -polar? Do SO 2, CO 2 and NO2 have the similar 3D structures? Polarity! 极性 Polar? Non -polar? 极性 非极性
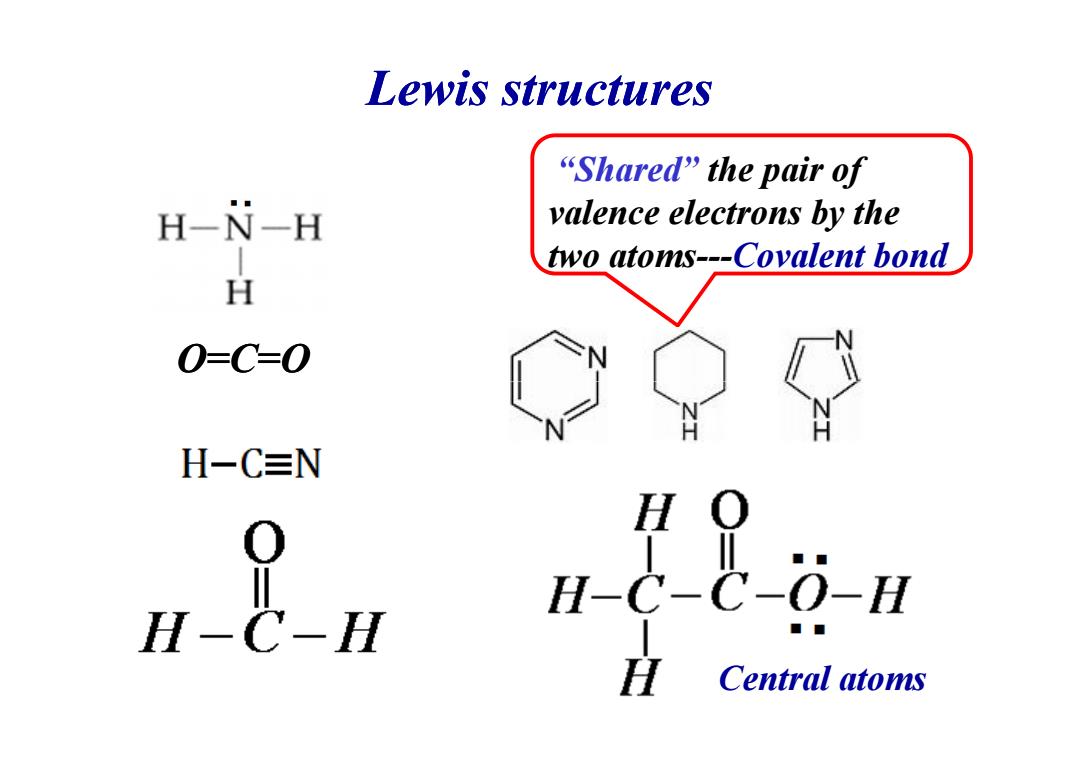
Lewis structures “Shared”the pair of H-N-H valence electrons by the two atoms---Covalent bond H 0=C=0 H-C≡N :人 应 Central atoms
Lewis structures O=C=O “Shared” the pair of valence electrons by the two atoms---Covalent bond Central atoms
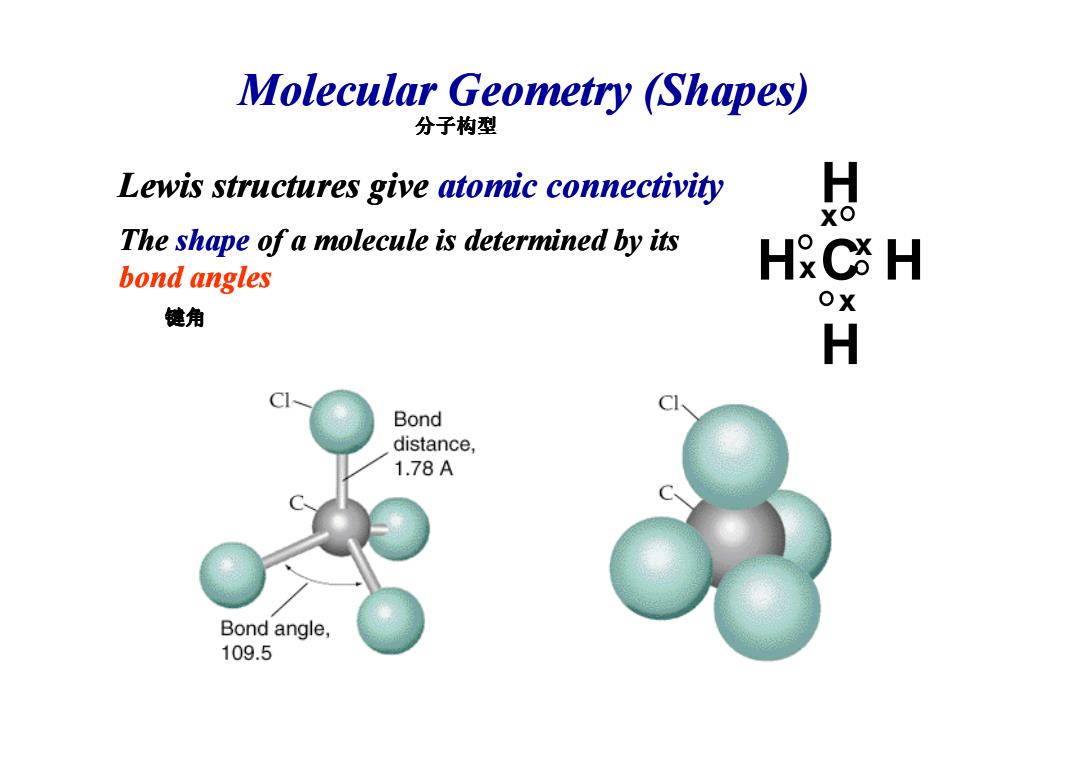
Molecular Geometry (Shapes) 分子构型 Lewis structures give atomic connectivity 如 The shape of a molecule is determined by its bond angles HC H 键角 ox H Bond distance, 1.78A Bond angle, 109.5
Molecular Geometry (Shapes) Lewis structures give atomic connectivity The shape of a molecule is determined by its bond angles H H H C Hx x x x 分子构型 键角