
Naming Inorganic Compounds Chapter 2
Naming Inorganic Compounds Chapter 2
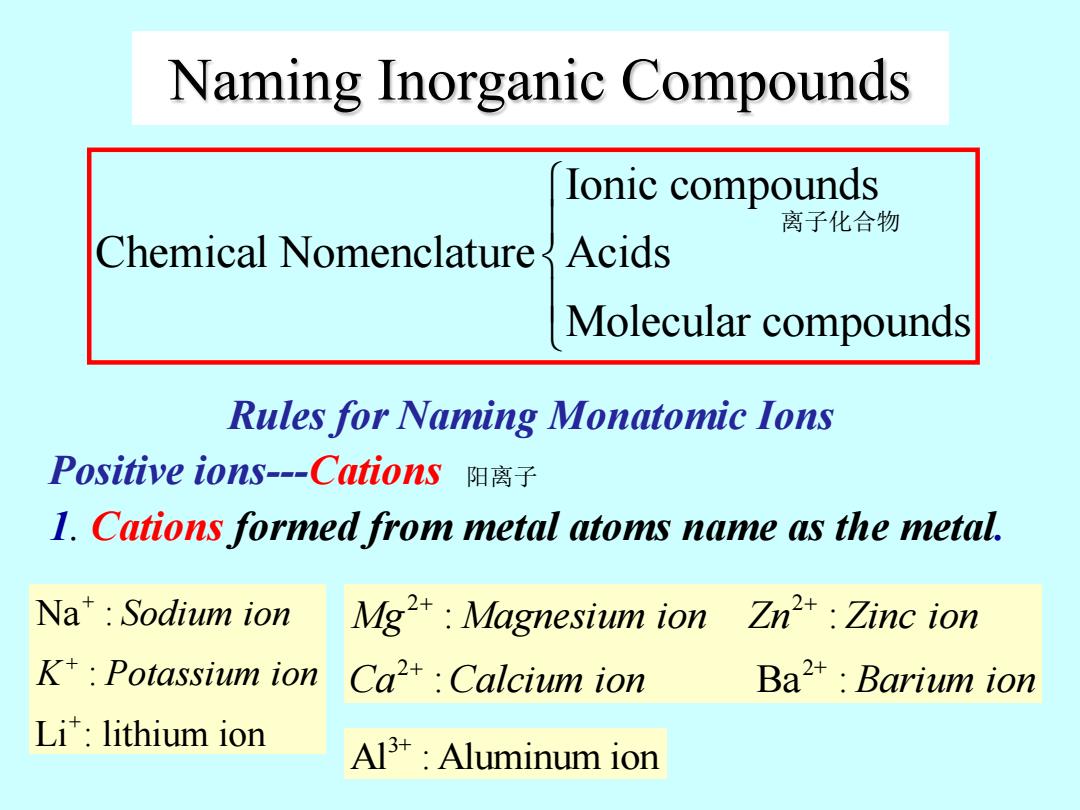
Naming Inorganic Compounds lonic compounds 离子化合物 Chemical Nomenclature Acids Molecular compounds Rules for Naming Monatomic Ions Positive ions--Cations阳离子 1.Cations formed from metal atoms name as the metal. Na*Sodium ion Mg:Magnesium ion Zn2:Zinc ion K*:Potassium ion Ca2+Calcium ion Ba2 Barium ion Li:lithium ion Al3+:Aluminum ion
Rules for Naming Monatomic Ions Positive ions---Cations 1. Cations formed from metal atoms name as the metal. Ionic compounds Chemical Nomenclature Acids Molecular compounds Naming Inorganic Compounds + + Na : : Li : lithium ion Sodium ion K Potassium ion 2 2 2 2+ : : : Ba : Mg Magnesium ion Zn Zinc ion Ca Calcium ion Barium ion 3+ Al : Aluminum ion 离子化合物 阳离子
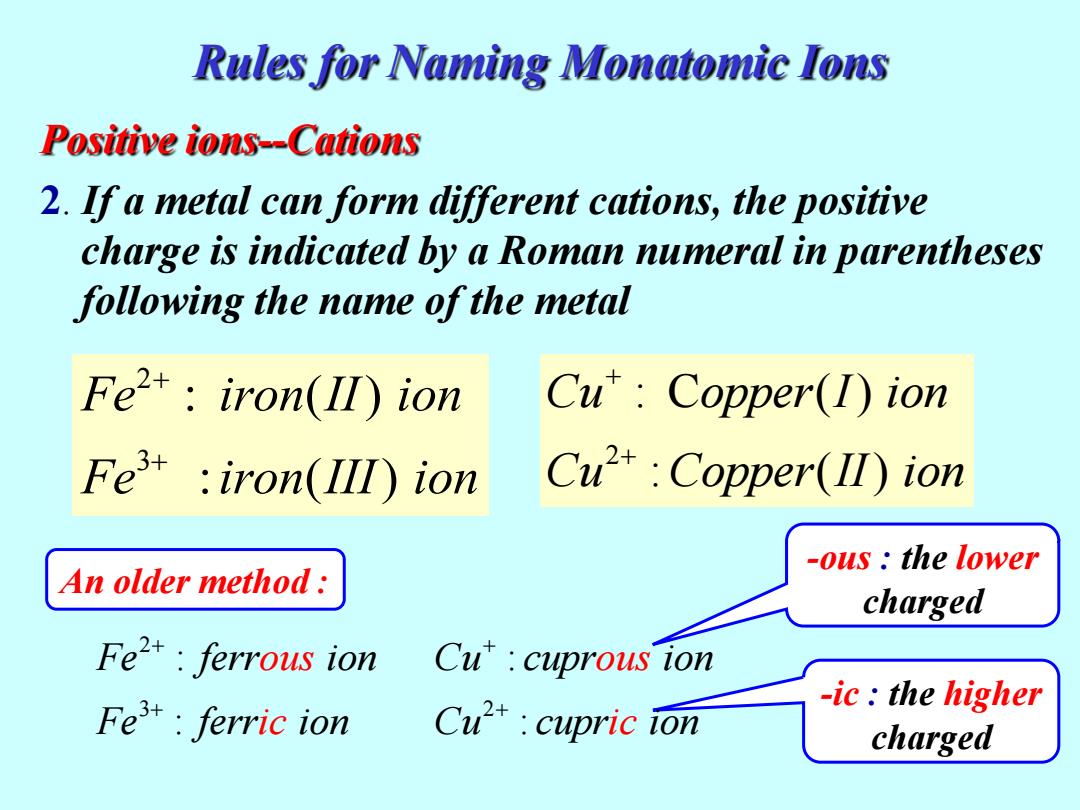
Rules for Naming Monatomic Ions Positive ions--Cations 2.If a metal can form different cations,the positive charge is indicated by a Roman numeral in parentheses following the name of the metal Fet:iron(II)ion Cu:Copper(1)ion Fe iron(III ion Cu2+:Copper(I)ion -ous the lower An older method: charged Fe2:ferrous ion Cu'cuprous ion -ic:the higher Fe:ferric ion Cu*cupric ion charged
Positive ions--Cations 2. If a metal can form different cations, the positive charge is indicated by a Roman numeral in parentheses following the name of the metal 2 3 : ( ) : ( ) Fe iron II ion Fe iron III ion + 2 : C ( ) : ( ) Cu opper I ion Cu Copper II ion -ous : the lower charged -ic : the higher charged An older method : Rules for Naming Monatomic Ions 2 3 : Fe ferr ion : Fe fer ous r n ic io 2 : : Cu cupr ion Cu cup ous r n ic io

Fe2:iron(II)ion Fe:iron(III ion cover oil● spray microscope several thousand volts uniform electric field Simplified scheme of Millikan's oil drop experiment
Simplified scheme of Millikan’s oil drop experiment 2 3 : ( ) : ( ) Fe iron II ion Fe iron III ion
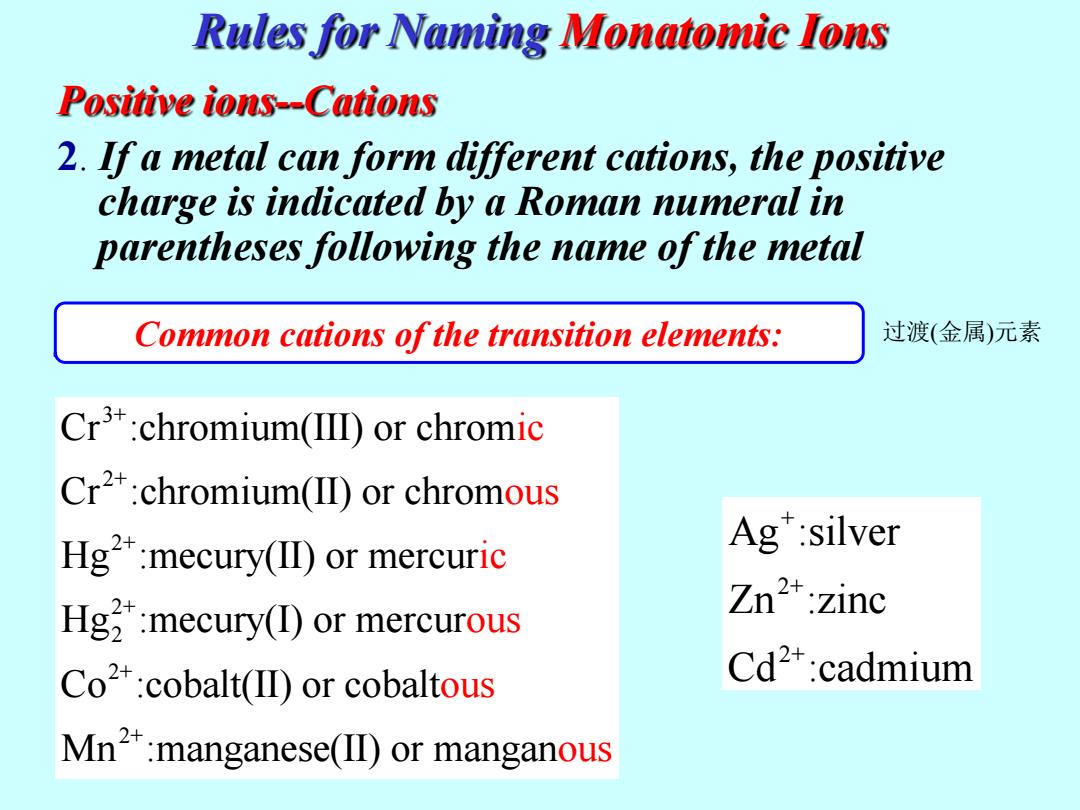
Rules for Naming Monatomie Ions Positive ions--Cations 2.If a metal can form different cations,the positive charge is indicated by a Roman numeral in parentheses following the name of the metal Common cations of the transition elements: 过渡(金属)元素 Cr3:chromium(IID)or chromic Cr2+:chromium(II)or chromous Hg2:mecury(II)or mercuric Ag":silver Hg:mecury(I)or mercurous Zn2+:zinc Co2+:cobalt(II)or cobaltous Cd2*:cadmium Mn2+:manganese(ID)or manganous
Positive ions--Cations 2. If a metal can form different cations, the positive charge is indicated by a Roman numeral in parentheses following the name of the metal Common cations of the transition elements: Rules for Naming Monatomic Ions 3+ 2+ 2+ 2+ 2 2+ 2+ Cr :chromium(III) or chrom Cr :chromium(II) or chrom Hg :mecury(II) or mercur Hg :mecury(I) or mercur Co :cobalt(II) or cobalt Mn :manganese ic ous ic ous ous (II) or manga nous + 2+ 2+ Ag :silver Zn :zinc Cd :cad mium 过渡(金属)元素

Rules for Naming Monatomic Ions Positive ions--Cations 3.Cations formed from nonmetal atoms have names that end in -ium NH:ammonium ion HO:hydronium ion
4 3 : NH ammon ion : H O hydro ium n n ium io Positive ions--Cations 3. Cations formed from nonmetal atoms have names that end in -ium Rules for Naming Monatomic Ions

Rules for Naming Ions Negative ions--Anions 1.The names of the monatomic anions are formed by replacing the end of the names of elements by -ide H H-hydrogen H:hydride ion 02 O-oxygen O2:oxide ion,:peroxide ion, S2- S-sulfur S2-:sulfide ion N3- N-nitrogen N:nitride ion F-fluorine F:fluoride ion Cl-chlorine CI:chloride ion Br Br-bromine Br:bromide ion I I-iodine I:iodide ion 2.A few simple polyatomic ions also have names ending in -ide OH:hydroxide ion, CN-:cyanide ion
H hydrogen O oxygen S sulfur N nitrogen Negative ions--Anions 1. The names of the monatomic anions are formed by replacing the end of the names of elements by –ide F fluorine Cl chlorine Br bromine I iodine Rules for Naming Ions 2 2 3 H O S N 2 2- 2 2 3 : : ; O : ; : : H hydr ion O ox ion perox ide ion S sulf i ide ide on N nitr ide ide ion F Cl Br I : : : : F fluor ion Cl chlor ion Br brom ion ide ide i I iod de ide ion 2. A few simple polyatomic ions also have names ending in –ide OH hydrox ion CN cyan : ; : ide ide i no

Rules for Naming Ions Negative ions--Anions 3.Polyatomic anions containing oxygen have names ending in -ate or -ite NO,:nitrate ion SO:sulfate ion NO,:nitrite ion SO:sulfite ion CIO:perchlorate ion Cl03: chlorate ion C102: chlorite ion ClO:hypochlorite ion
Negative ions--Anions 3. Polyatomic anions containing oxygen have names ending in –ate or –ite 2 4 2 3 : : SO sulf ion SO sulf ate ite ion Rules for Naming Ions 3 2 : NO nitr ion : NO nitr ate ite ion 4 3 2 : : : : ClO chlor ion ClO chlor ion ClO chlor io per ate ate ite h n ClO chlor ypo ite ion
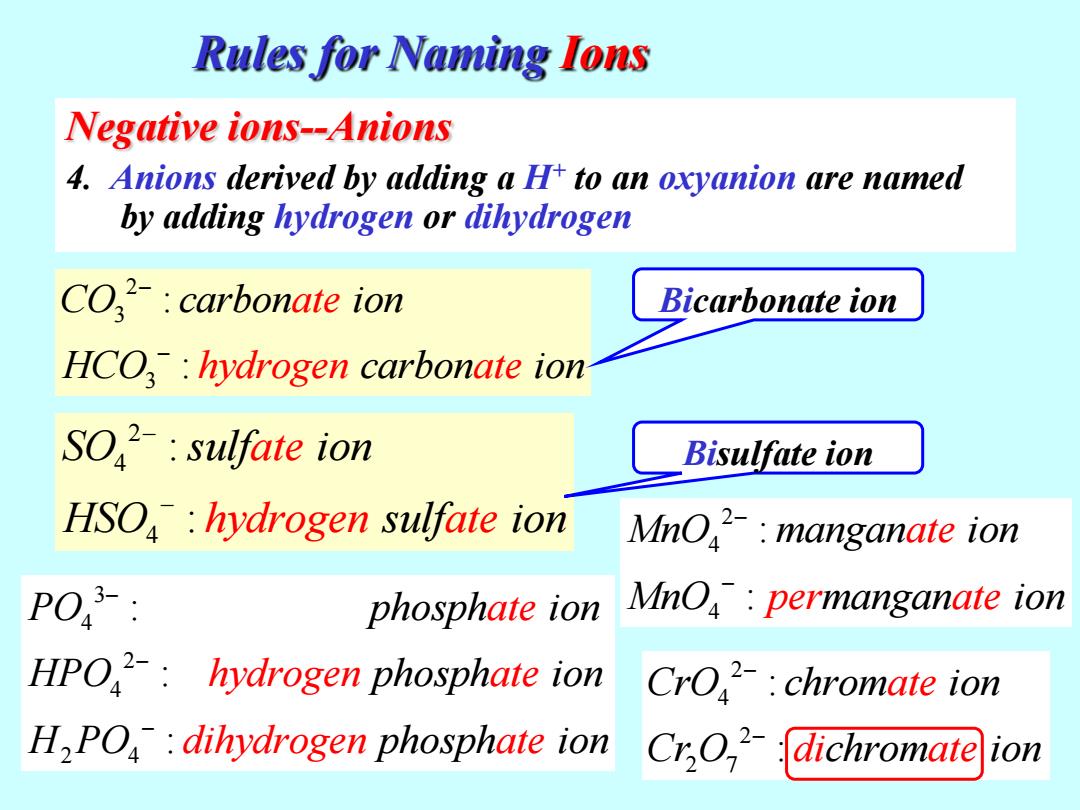
Rules for Naming Ions Negative ions--Anions 4.Anions derived by adding a H to an oxyanion are named by adding hydrogen or dihydrogen CO-:carbonate ion Bicarbonate ion HCO:hydrogen carbonate ion SO:sulfate ion Bisulfate ion HSO:hydrogen sulfate ion MnO:manganate ion PO phosphate ion MnO:permanganate ion HPO:hydrogen phosphate ion CrO:chromate ion H,PO:dihydrogen phosphate ion Cr,O:dichromate ion
Negative ions--Anions 4. Anions derived by adding a H+ to an oxyanion are named by adding hydrogen or dihydrogen 2 4 4 : : S ate hydrogen O sulf ion HSO sulf o ate i n Rules for Naming Ions 2 3 3 : : C ate hydrogen O carbon ion HCO carbon n ate io Bisulfate ion Bicarbonate ion 3 4 2 4 2 4 : : : ate hy PO phosph ion HPO phosph ion H PO drogen ate dihydrogen at phosph io e n 2 4 2 2 7 : : CrO chrom ion Cr O chrom ate di ate ion 2 4 4 : : MnO mangan ion MnO mangan ate per ate ion

Rules for Naming Ionic Compounds Ionic Compounds name of cation name of anion NaCl:sodium chloride CaCh calcium chloride Al(NO):aluminum nitrate Cu(ClO)2:copper(I)perchlorate cupric perchlorate Ba(OH),:Barium hydroxide NH,Br:ammonium bromide CrO:chromium(III)oxide
Ionic Compounds name of cation + name of anion Rules for Naming Ionic Compounds 2 : : NaCl CaCl 3 3 4 2 2 ( ) : ( ) : ( ) : Al NO Cu ClO Ba OH2 : : NaCl sodium chlor CaCl calcium chl ide oride 3 3 4 2 2 ( ) : ( ) : ( ) ( ) : Al NO alumin ate per ate p um nitr Cu ClO copper II chlor cupric chlor Ba OH Barium hydro er ate xide 4 2 3 : : NH Br Cr O 4 2 3 : : ( ) NH Br ammon brom Cr O chromium ium id III o e xide