
Homework-Chap.9 1. EOC (end-of-chapter exercises): 9.21,9.26,9.36,9.38,9.48,9.56, 2.Team work report(4 persons/group): Self learning,answering Questions Discuss together: Reading materials:Chemistry at work---Organic Dye p385 a.What are HUMO and LUMO? b.Why some substances,such as organic dyes,are colorful?briefly explain the reasons according to what you have learnt from "Organic dyes"(in the light of molecular orbital theory).What kinds oforganic compounds are typical colorful materials? c.What are the delocalized n electrons and delocalized n bonds? d.Answer the question of 9.93 9.104(f); e.List the questions which you didn't understand after your learning and discussion. (Both reports in English or Chinese are acceptable) 3.Read summary and key terms and write down new words and key terms on your notebook (9.1-9.6)
Homework-Chap.9 1. EOC (end EOC (end-of-chapter exercises): chapter exercises): 9.21, 9.26, 9.36, 9.38, 9.48, 9.56, 2. Team work report (4 persons/group) Team work report (4 persons/group): Self learning, answering Questions & Discuss together: Reading materials: Chemistry at work Reading materials: Chemistry at work---Organic D Organic Dye p385 ye p385 a. What are HUMO and LUMO? b. Why some substances, such as organic dyes, are colorful? briefly explain the reasons according to what you have learnt from “Or reasons according to what you have learnt from “Organic dyes ganic dyes” (in the light of the light of molecular orbital theory). What kinds of organic compounds are typical colorful materials? c. What c. What are the delocalized are the delocalized π electrons and delocalized electrons and delocalized π bonds? d. Answer the question of 9.93 & 9.104(f); Answer the question of 9.93 & 9.104(f); e. List the questions which you didn’t understan List the questions which you didn’t understand after your learning learning and discussion. (Both reports in English or Chinese are acceptable) 3. Read summary and key terms and write down new words and key rite down new words and key terms on your notebook (9.1-9.6)

(电子排布式) Review Electron Configuration in Molecular Orbitals and Bond Order(睫领) ☐1s H atom Hatom H atom H atom He atom He atom He atom He atom HMolecule H:molecule He:Molecule He,Molecule Electron configuration O\s RG2ua 51s 1s 1s H克 H2 He He2 bond order: 归 1 h 0
Review : Electron Configuration Review : Electron Configuration in Molecular Orbitals and Bond Order Bond Order (键级) 2 *2 σ σ 1 σ1s 2 σ1s 2 *1 σ σ Electron configuration (电子排布式) bond order: ½ 1 0 ½ 2 * 1s 2 σ σ 1s σ1s σ1s 2 * 1s 1 σ σ1s configuration
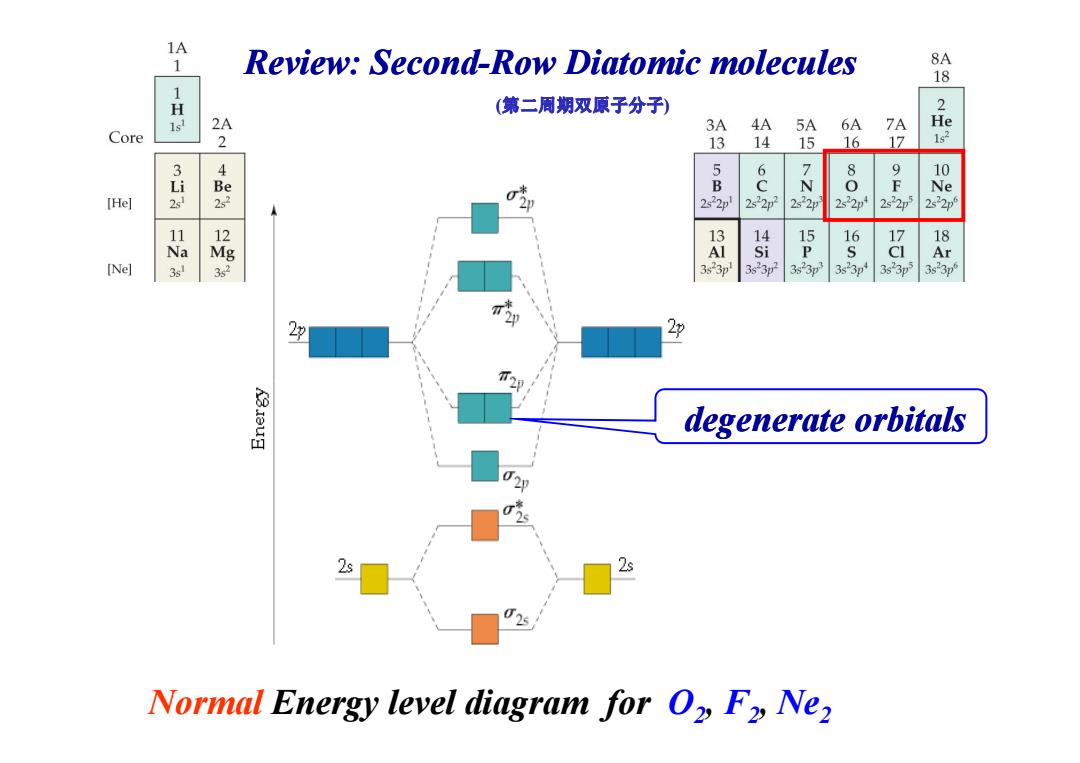
1A 1 Review:Second-Row Diatomic molecules 8A 18 H (第二周期双原子分子) 2 2A 3A 4A 5A 6A 7A He Core 2 13 14 15 16 17 1s2 6 8 9 10 i Be B N F Ne [He] 2s22p 2s22p72 2s22p 2s22p1 2s22p 2522p 13 15 16 17 18 Na Mg i P S Ar [Ne] 3sl 2 3s23p 3623p 3s23p3 3s23p 3s23p5 3s23p 2 degenerate orbitals 2s 2s Normal Energy level diagram for O2 F2 Ne2
Review: Second Review: Second-Row Diatomic molecules Diatomic molecules (第二周期双原子分子) Normal Energy level diagram for O2, F2, Ne2 degenerate degenerate orbitals orbitals
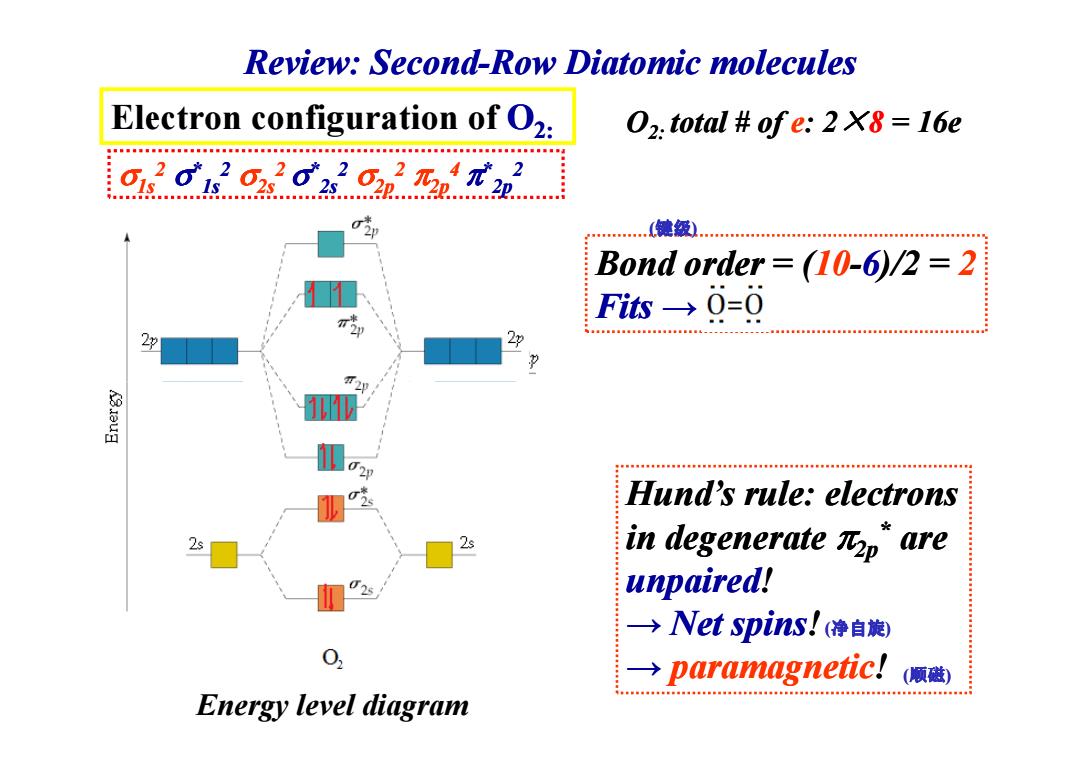
Review:Second-Row Diatomic molecules Electron configuration of O2: 02.total ofe:2 X8 16e 01 615 …(键级 Bond order (10-6)/2 =2 Fis→0=0 Hund's rule:electrons 2s in degenerate Rp"are unpaired! →Net spins!(净自旋) → paramagnetic! (顺磁) Energy level diagram
Electron configuration of O2: σ1s2 σ*1s2 σ2s2 σ*2s2 σ2p2 π2p4 π*2p2 Bond order = (10-6)/2 = 2 Fits → O2: total # total # of e: 2 × 8 = 16e (键级) Review: Second Review: Second-Row Diatomic molecules Diatomic molecules Hund’s rule: electrons in degenerate π2p* are unpaired! → Net spins Net spins! → paramagnetic! Energy level diagram (净自旋) (顺磁)
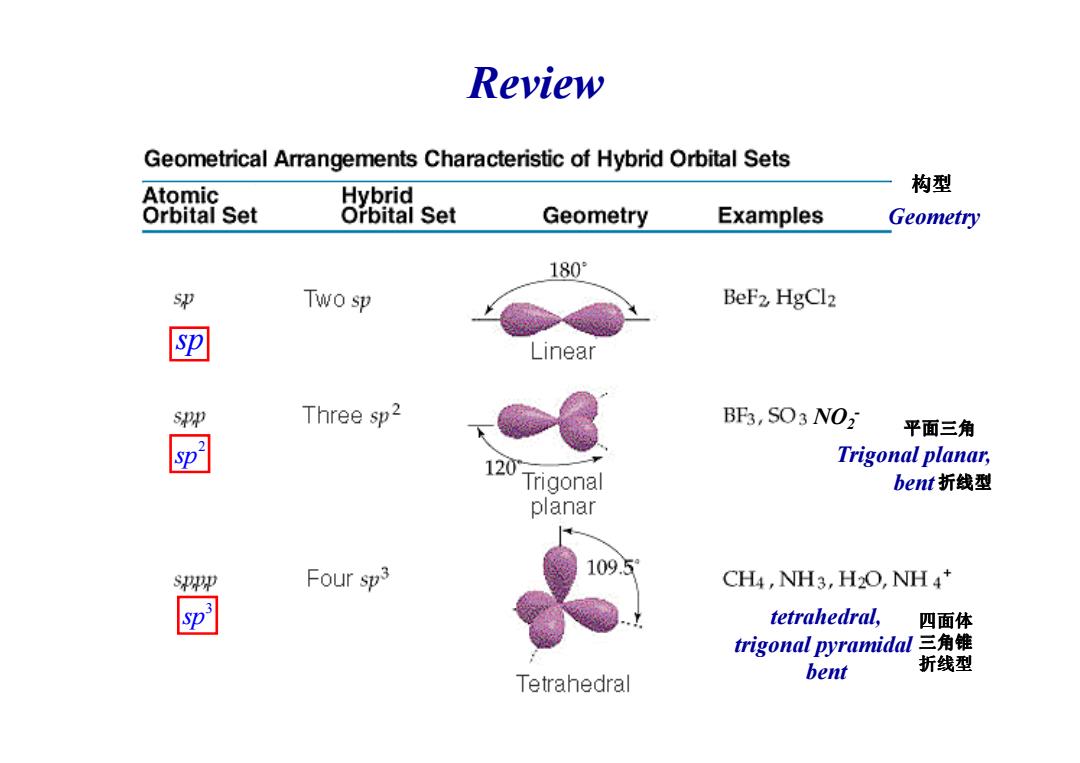
Review Geometrical Arrangements Characteristic of Hybrid Orbital Sets Atomic Hybrid 构型 Orbital Set Orbital Set Geometry Examples Geometry 180° Two sp BeF2 HgCl2 匣 Linear spp Three sp2 BF3,SO3 NO2 平面三角 120 Trigonal planar, Trigonal bent折线型 planar SPEp Four sp3 109.5 CH4,NH3,H2O,NH4* 3 sp tetrahedral, 四面体 trigonal pyramidal三角锥 折线型 Tetrahedral bent
Review sp Geometry 构型 2 sp 3 sp NO2- Trigonal planar, bent tetrahedral, trigonal pyramidal bent 平面三角 折线型 四面体 三角锥 折线型

Review Geometrical Arrangements Characteristic of Hybrid Orbital Sets Atomic Hybrid 构型 Orbital Set Orbital Set Geometry Examples Geometry 平面三角形 Trigonal bipyramidal, d Five sp PFs,SF4,BrF3 Seesaw跷跷板 sp'd T shape T-形 120° The elements in period Trigonal 3 or beyond with empty bipyramidal d orbitals 八面体 Octahedral, sypp,d Six sp2 SF6,CIFs,XeF4,PF 90 Square pyramidal sp'd- Square planar octahedral 四角锥 平面正方形
Review 3 sp d Trigonal Geometry Trigonal bipyramidal, Seesaw T shape 跷跷板 平面三角形 构型 T-形 The elements in period 3 2 sp d octahedral Trigonal bipyramidal Octahedral, Square pyramidal Square planar 八面体 四角锥 平面正方形 The elements in period 3 or beyond with empty d orbitals
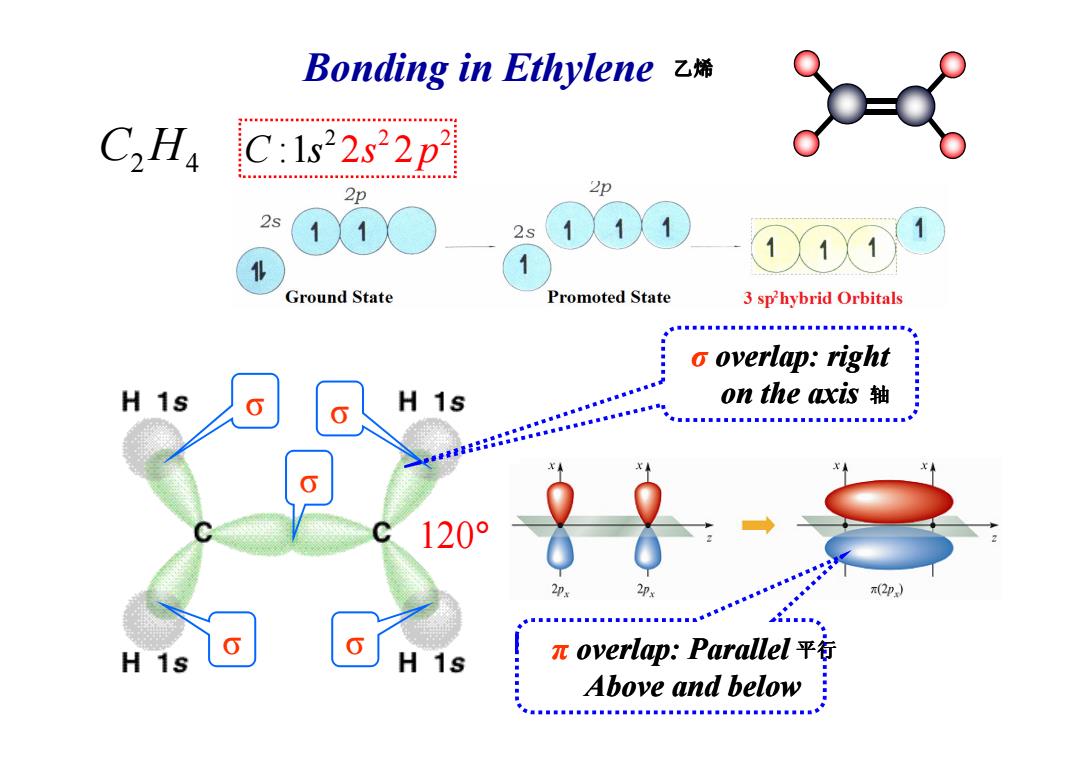
Bonding in Ethylene乙烯 C2 Ha C:1s22s22p 2p 2p 2s 2s 11 Ground State Promoted State 3 sphybrid Orbitals o overlap:right H 1s 222.0aa00 H 1s on the axis轴 120° 2px x(2p) 年■■■■■■■■■■■■■■■■■g。装装地着 H 1s H 1s πoverlap:Parallel平行 Above and below c.........c...s
Bonding in Ethylene C H2 4 2 2 2 C s :1 2 2 s p σ overlap: right on the axis 乙烯 σ σ σ σ σ 120 ° on the axis π overlap: Parallel Above and below 平行 轴
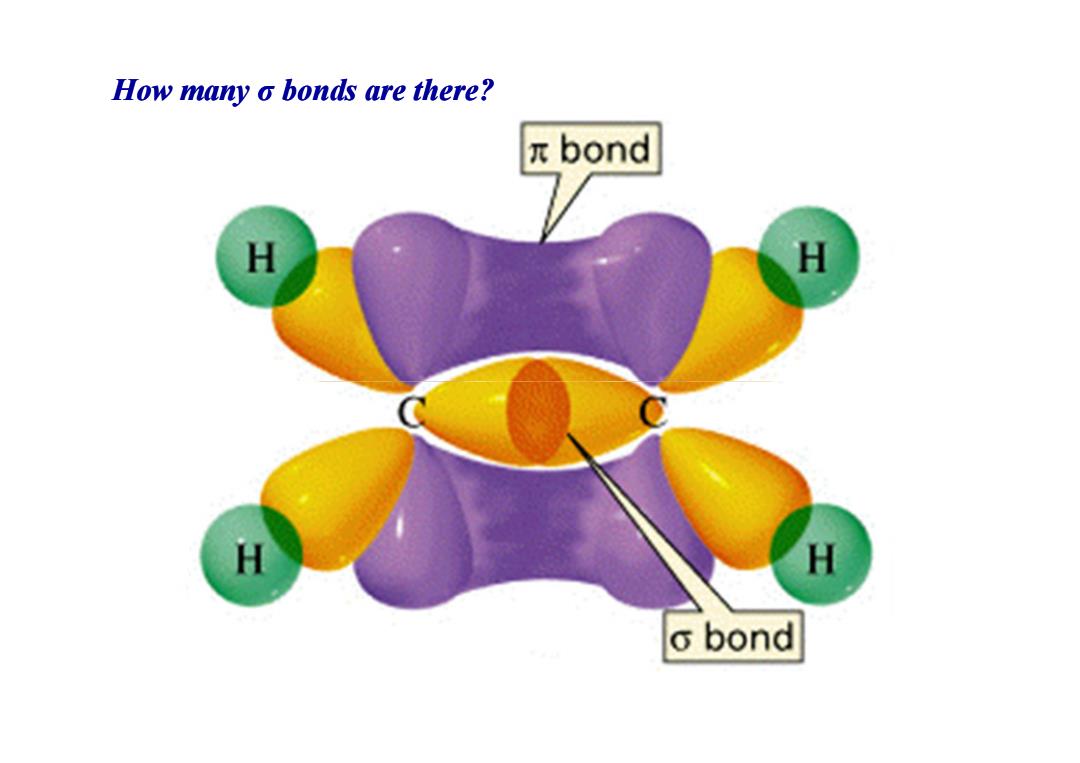
How many o bonds are there? πbond H H H H o bond
How many How many σ bonds are there? bonds are there?
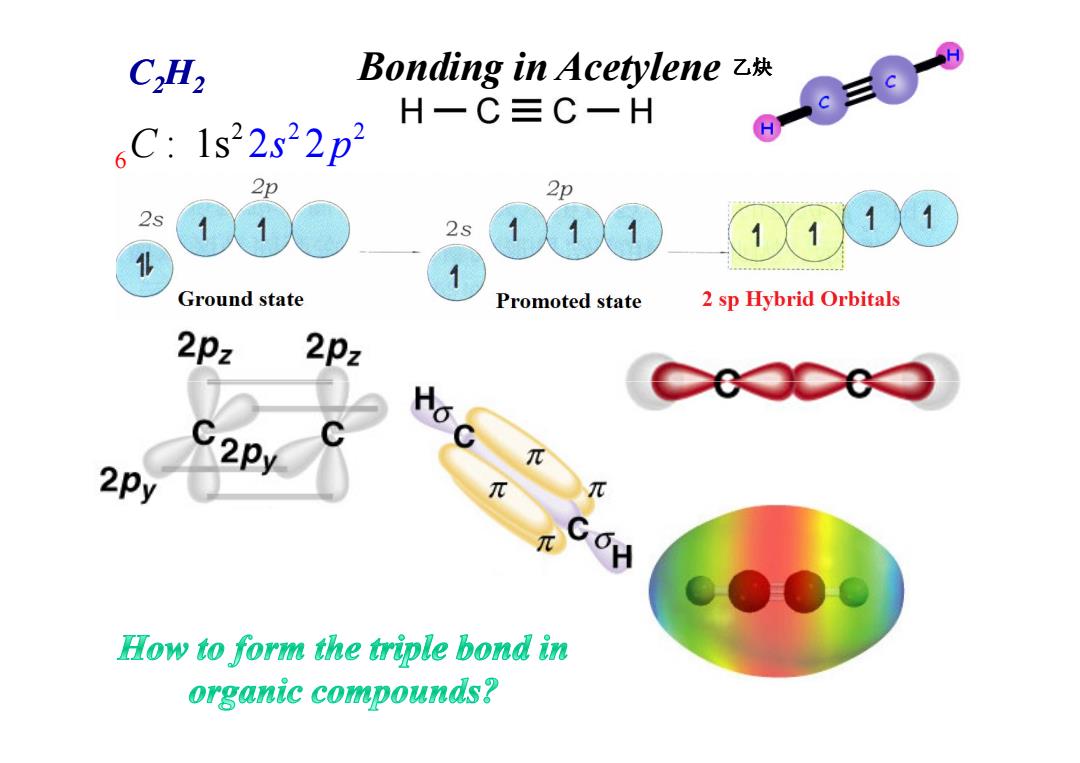
C2H2 Bonding in Acetylene z块 H一C三C一H ,C:1s22s22p2 2p 2p 2s 2s 1 1 人1X 1 Ground state Promoted state 2 sp Hybrid Orbitals 2pz 2pz C2Py 6 2Py How to form the triple bond in organic compounds?
Bonding in Acetylene 2 6 2 2 C : 1s 2s p 2 C2H2 乙炔
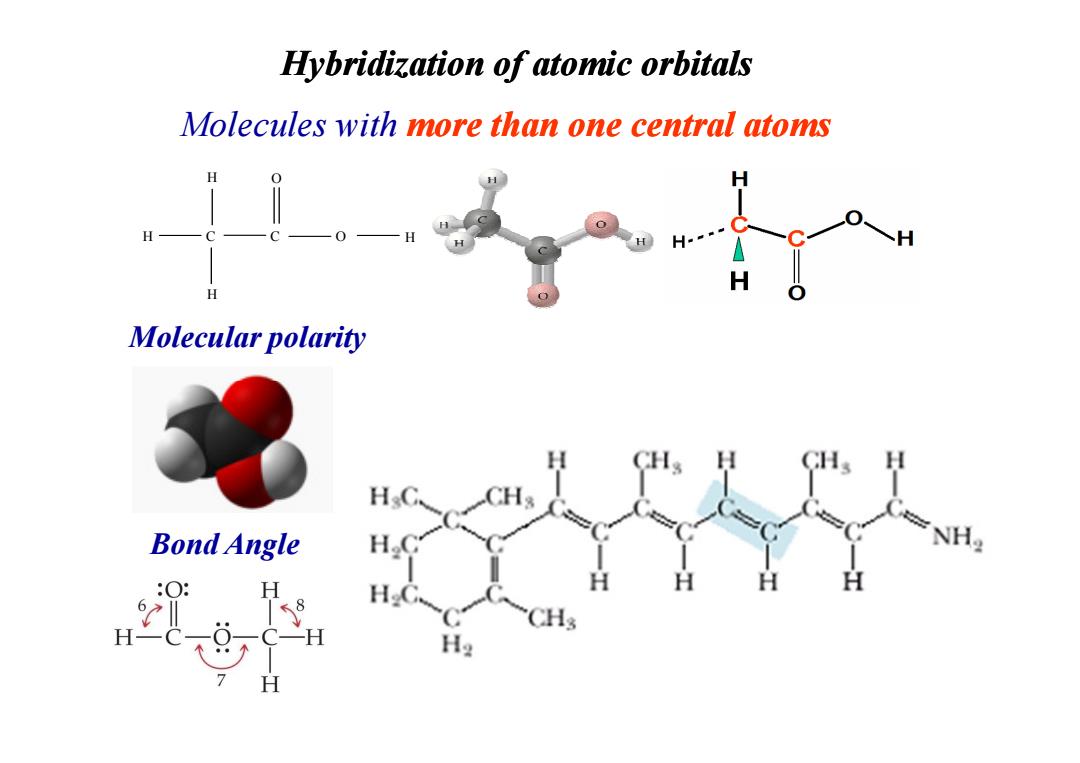
Hybridization of atomic orbitals Molecules with more than one central atoms H H C H H-= H H Molecular polarity CH H Bond Angle NH2 :O H ≤8 H2
Hybridization of atomic Hybridization of atomic orbitals orbitals Molecules with more than one central atoms H H C O O H H C Molecular polarity Bond Angle