正在加载图片...
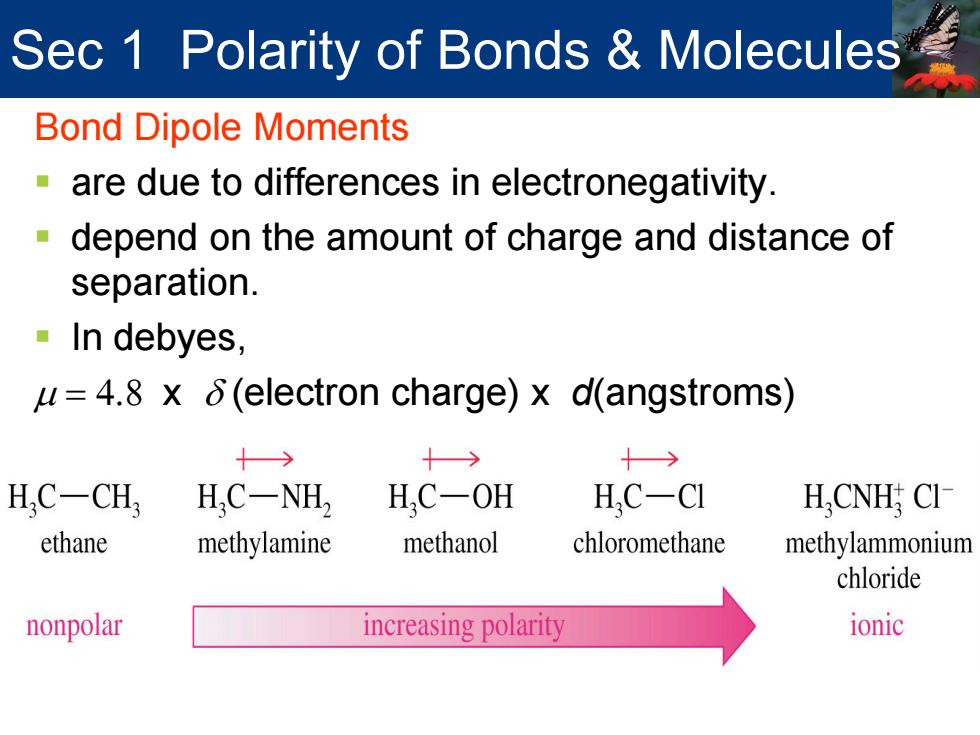
Sec 1 Polarity of Bonds Molecules Bond Dipole Moments are due to differences in electronegativity. -depend on the amount of charge and distance of separation. In debyes, u=4.8 x 6(electron charge)x d(angstroms) 十→ 十→ H.C-CH, H,C-NH2 H,C-OH H.C-CI H,CNH CI ethane methylamine methanol chloromethane methylammonium chloride nonpolar increasing polarity ionic Sec 1 Polarity of Bonds & Molecules Bond Dipole Moments are due to differences in electronegativity. depend on the amount of charge and distance of separation. In debyes, µ = 4.8 x δ (electron charge) x d(angstroms)