正在加载图片...
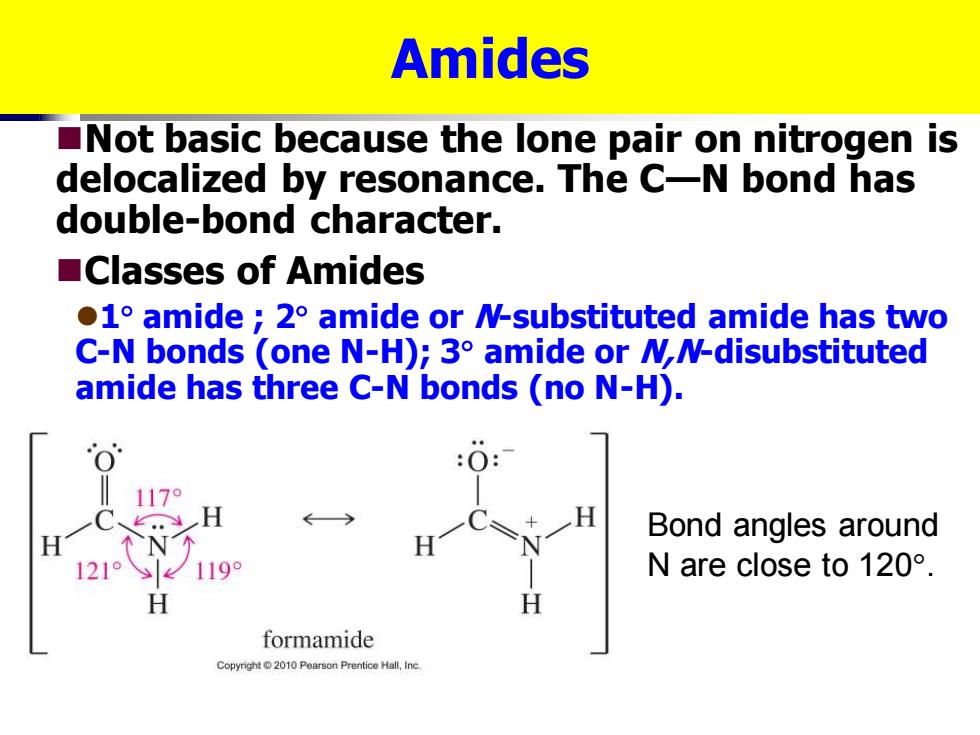
Amides Not basic because the lone pair on nitrogen is delocalized by resonance.The C-N bond has double-bond character. ■Classes of Amides 1 amide 2 amide or N-substituted amide has two C-N bonds (one N-H);3 amide or MN-disubstituted amide has three C-N bonds (no N-H). 170 Bond angles around 1219 1199 N are close to120°. H formamide Copyright2010 Pearson Prentice Hall.Inc. Amides ◼Not basic because the lone pair on nitrogen is delocalized by resonance. The C—N bond has double-bond character. ◼Classes of Amides ⚫1 amide ; 2 amide or N-substituted amide has two C-N bonds (one N-H); 3 amide or N,N-disubstituted amide has three C-N bonds (no N-H). Bond angles around N are close to 120