正在加载图片...
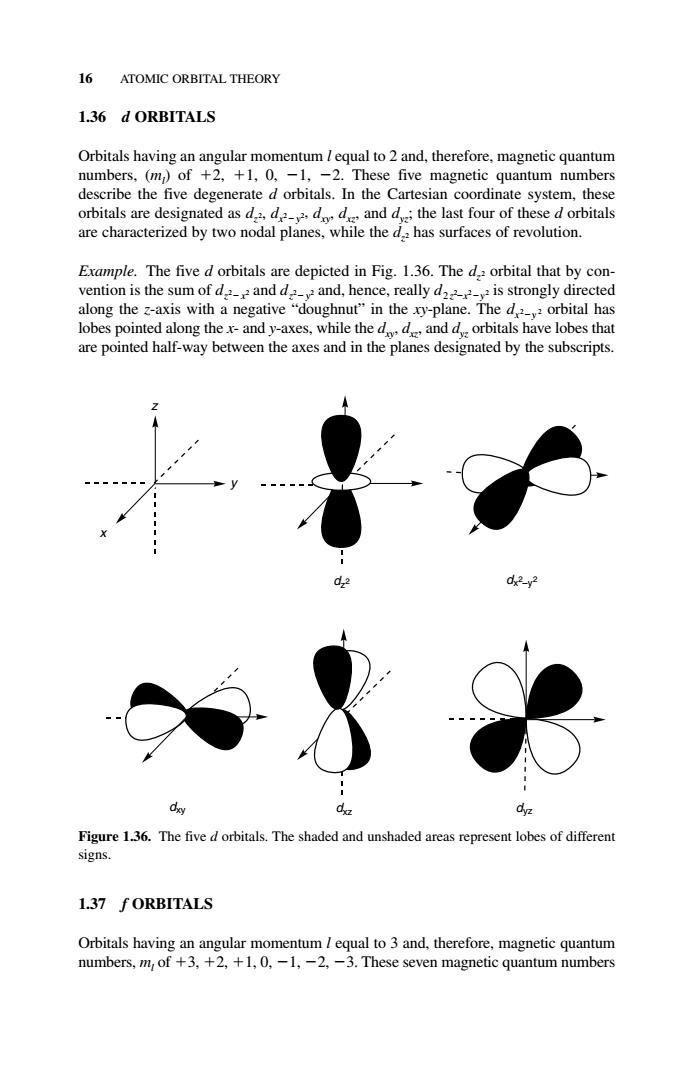
16 ATOMIC ORBITAL THEORY 1.36 d ORBITALS Orbitals having an angular momentum /equal to 2 and,therefore,magnetic quantum numbers,(m of +2.+1,0.-1,-2.These five magnetic quantum numbers describe the five degenerate d orbitals.In the Cartesian coordinate system,these orbitals are desig ated as de d and d:the last four of the d orbitals are characterized by two nodal planes. hil thehas surfaces of revolution. Example.The five d orbitals are depicted in Fig.1.36.The d orbital that by con- vention is the sum of d and d and,hence,really d is strongly directed along the z-axis with a negative"doughnut"in the xy-plane.The dorbital has lobes pointed along thex-and y-axes,while the ddanddorbitals have lobes that are pointed half-way between the axes and in the planes designated by the subscripts 02 d Figure 1.36.The five d orbitals.The shaded and unshaded areas represent lobes of different signs. 1.37 fORBITALS Orbitals having an angular momentum equal to 3 and,therefore,magnetic quantum numbers,m of +3,+2,+1,0,-1,-2,-3.These seven magnetic quantum numbers 1.36 d ORBITALS Orbitals having an angular momentum l equal to 2 and, therefore, magnetic quantum numbers, (ml ) of 2, 1, 0, 1, 2. These five magnetic quantum numbers describe the five degenerate d orbitals. In the Cartesian coordinate system, these orbitals are designated as dz2, dx2 y2, dxy, dxz, and dyz; the last four of these d orbitals are characterized by two nodal planes, while the dz2 has surfaces of revolution. Example. The five d orbitals are depicted in Fig. 1.36. The dz2 orbital that by convention is the sum of dz2 x2 and dz2 y2 and, hence, really d2 z2 x2 y2 is strongly directed along the z-axis with a negative “doughnut” in the xy-plane. The dx2 y 2 orbital has lobes pointed along the x- and y-axes, while the dxy, dxz, and dyz orbitals have lobes that are pointed half-way between the axes and in the planes designated by the subscripts. 1.37 f ORBITALS Orbitals having an angular momentum l equal to 3 and, therefore, magnetic quantum numbers, ml of 3, 2, 1, 0, 1, 2, 3. These seven magnetic quantum numbers 16 ATOMIC ORBITAL THEORY y z x dz2 dx2−y2 dxy dxz dyz Figure 1.36. The five d orbitals. The shaded and unshaded areas represent lobes of different signs. c01.qxd 5/17/2005 5:12 PM Page 16�����