正在加载图片...
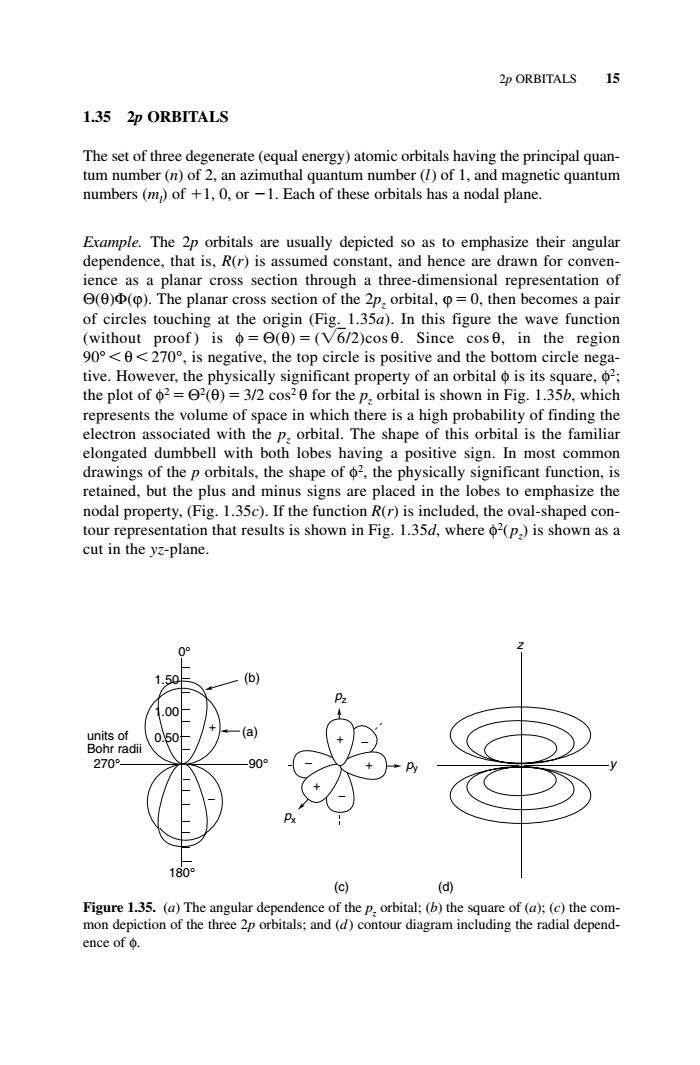
2p ORBITALS 15 1.35 2p ORBITALS The set of three degenerate(equal energy)atomic orbitals having the principal quan tum number(n)of 2,an azimuthal quantum number(/)of 1,and magnetic quantum numbers (m)of +1,0,or-1.Each of these orbitals has a nodal plane. Erample.The 2p orbitals are usually depicted so as to emphasize their angular dependence,that is,R(r)is assumed constant,and hence are drawn for co e as a plana section through a th ree-dim .The ensionalrepresentaio of planar cross section of the P:orbita then becomesa pair of circles touching at the origin (Fig.1.35a).In this figure the wave function (without proof)is =e()=(V6/2)cos 0.Since cos6,in the region 90<0<270,is negative,the top circle is positive and the bottom circle nega- tive.However.the physically significant property of an orbital o is its square.2; the plot of2=日2(θ)=3/2cos20 for the p,orbital is shown in Fig.1.35b.which the volume of pace in which there ig probab of finding tho rbital.The shape of this orbital is the fan nilia ongated 0eiedwid bbell ith both obes sign. comm drawing ction,I nodal property,(Fig.1.35c).If the function R(r)is included,the oval-shaped con- tour representation that results is shown in Fig.1.35d,where 2(p,)is shown as a cut in the yz-plane (d Figure 1.35.(a)T o the p.orbital:( (B)the s 2p orbitals;and (d)c ng the ra ace of。1.35 2p ORBITALS The set of three degenerate (equal energy) atomic orbitals having the principal quantum number (n) of 2, an azimuthal quantum number (l) of 1, and magnetic quantum numbers (ml ) of 1, 0, or 1. Each of these orbitals has a nodal plane. Example. The 2p orbitals are usually depicted so as to emphasize their angular dependence, that is, R(r) is assumed constant, and hence are drawn for convenience as a planar cross section through a three-dimensional representation of Θ(θ)Φ(ϕ). The planar cross section of the 2pz orbital, ϕ 0, then becomes a pair of circles touching at the origin (Fig. 1.35a). In this figure the wave function (without proof ) is φ Θ(θ) (6/2)cos θ. Since cos θ, in the region 90° θ 270°, is negative, the top circle is positive and the bottom circle negative. However, the physically significant property of an orbital φ is its square, φ2; the plot of φ2 Θ2(θ) 3/2 cos2 θ for the pz orbital is shown in Fig. 1.35b, which represents the volume of space in which there is a high probability of finding the electron associated with the pz orbital. The shape of this orbital is the familiar elongated dumbbell with both lobes having a positive sign. In most common drawings of the p orbitals, the shape of φ2, the physically significant function, is retained, but the plus and minus signs are placed in the lobes to emphasize the nodal property, (Fig. 1.35c). If the function R(r) is included, the oval-shaped contour representation that results is shown in Fig. 1.35d, where φ2(pz) is shown as a cut in the yz-plane. 2p ORBITALS 15 (d) y z 0.50 1.00 1.50 90° − 180° 270° 0° units of Bohr radii + (a) (b) (c) px pz py + + + − − − Figure 1.35. (a) The angular dependence of the pz orbital; (b) the square of (a); (c) the common depiction of the three 2p orbitals; and (d) contour diagram including the radial dependence of φ. c01.qxd 5/17/2005 5:12 PM Page 15��