正在加载图片...
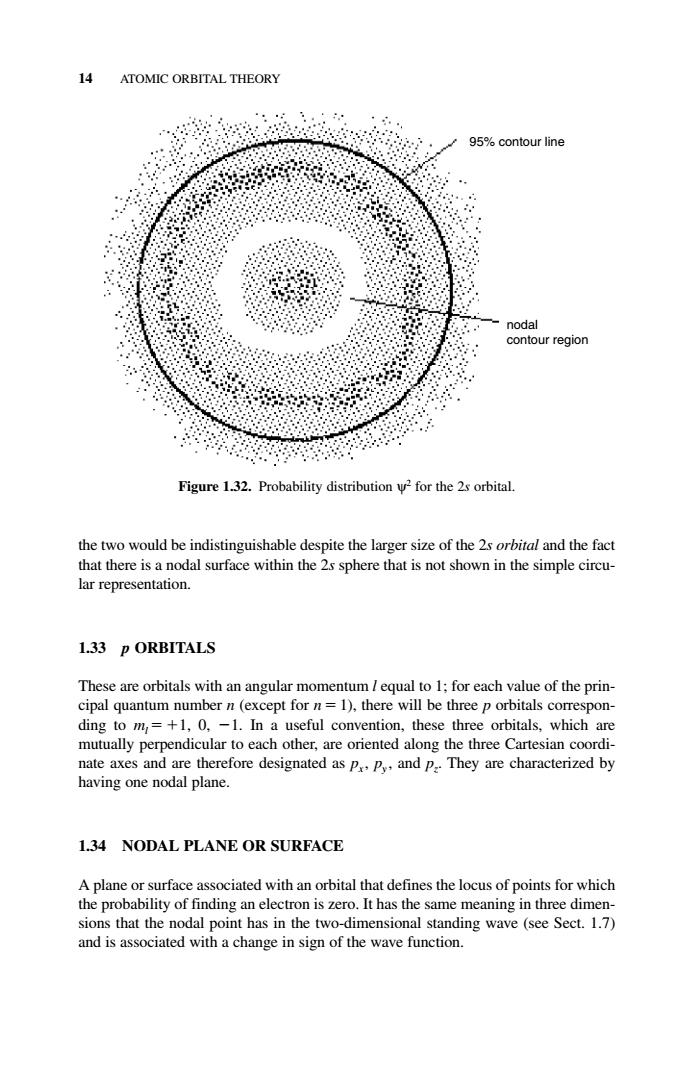
4 ATOMIC ORBITAL THEORY 95%contour line Figure 1.32.Probability distribution for the 2s orbital the two would be indistinguishable despite the larger size of the 2s orbital and the fact that there is a nodal surface within the 2s sphere that is not shown in the simple circu- lar representation. 1.33 p ORBITALS These are orbitals with an angular cipal quantum num ding to m=+1,0.-1.In a useful convention,these three orbitals,which are mutually perpendicular to each other,are oriented along the three Cartesian coordi- nate axes and are therefore designated as pp.and pThey are characterized by having one nodal plane. 1.34 NODAL PLANE OR SURFACE n is zero.It h noda and is associated with a change in sign of the wave function. the two would be indistinguishable despite the larger size of the 2s orbital and the fact that there is a nodal surface within the 2s sphere that is not shown in the simple circular representation. 1.33 p ORBITALS These are orbitals with an angular momentum l equal to 1; for each value of the principal quantum number n (except for n 1), there will be three p orbitals corresponding to ml 1, 0, 1. In a useful convention, these three orbitals, which are mutually perpendicular to each other, are oriented along the three Cartesian coordinate axes and are therefore designated as px , py , and pz . They are characterized by having one nodal plane. 1.34 NODAL PLANE OR SURFACE A plane or surface associated with an orbital that defines the locus of points for which the probability of finding an electron is zero. It has the same meaning in three dimensions that the nodal point has in the two-dimensional standing wave (see Sect. 1.7) and is associated with a change in sign of the wave function. 14 ATOMIC ORBITAL THEORY nodal contour region 95% contour line Figure 1.32. Probability distribution ψ2 for the 2s orbital. c01.qxd 5/17/2005 5:12 PM Page 14�