正在加载图片...
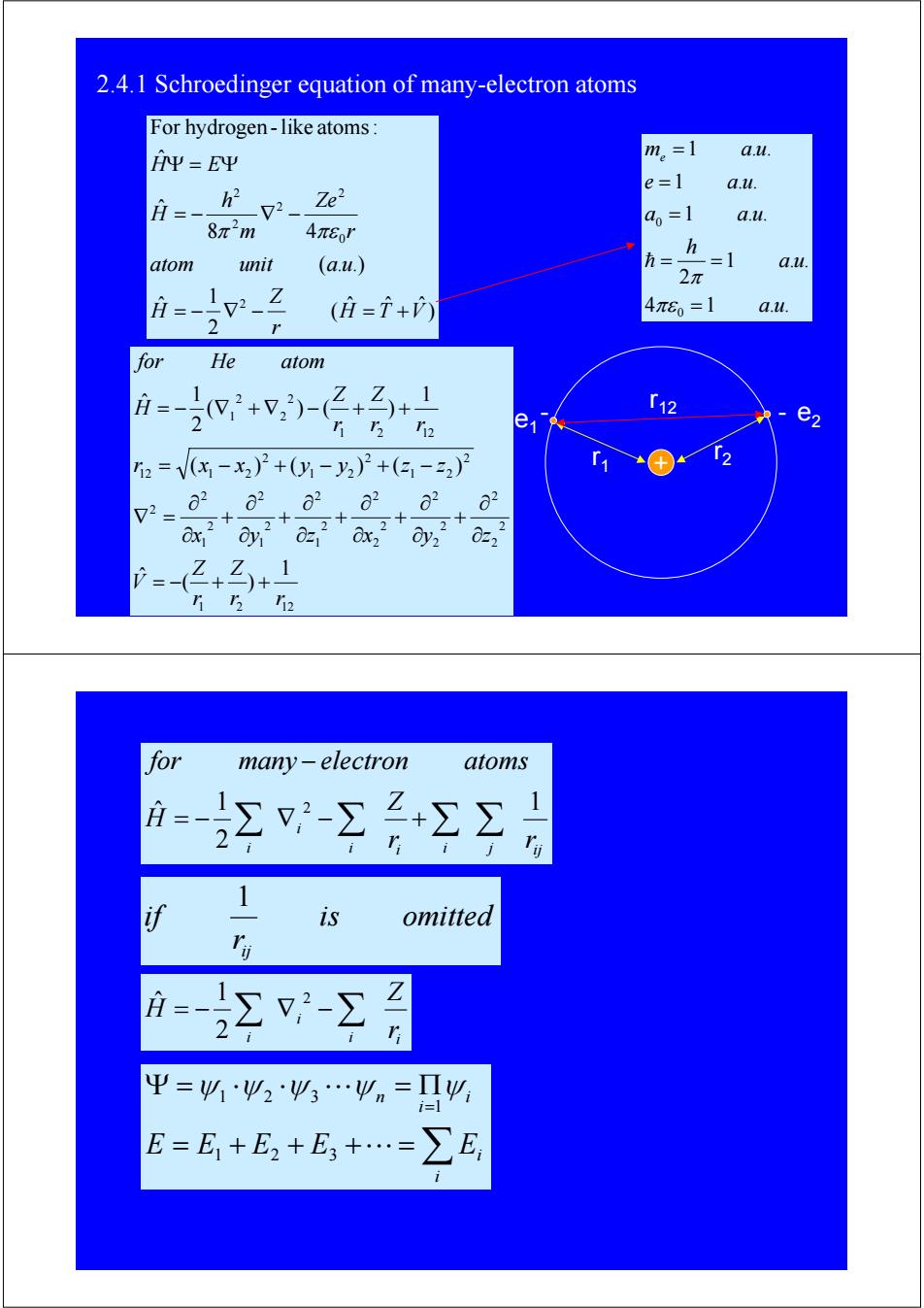
2.4.1 Schroedinger equation of many-electron atoms For hydrogen-like atoms: 平=E平 m。=1 au. e=1 au. = h2 Ze2 a=1 a.u. 8n'm 4πEo' h atom unit (a.u.) h二 =1 a.u. 2π 月=-12- 1 Z 4π60=1 a.u. 2 (庄=+內 for He atom T12 e1% e2 2=V(x-x2)2+0y-y2)2+(3,-22)》2 a2a22a2 2 ax y =-2+名)+1 12i2 for many-electron atoms =- Σ-工+Σ习 1- 1 is omitted 日Σ-Σ 平=41必2必3…9n=Π必 i=1 E=E+E2+E,+…=∑E2.4.1 Schroedinger equation of many-electron atoms e2 + e - - 1 r2 r12 r1 ) ˆ ˆ ˆ ( 2 1 ˆ ( . .) 8 4 ˆ ˆ For hydrogen -like atoms: 2 0 2 2 2 2 H T V r Z H atom unit a u r Ze m h H H E = − ∇ − = + = − ∇ − Ψ = Ψ π πε 4 1 . . 1 . . 2 1 . . 1 . . 1 . . 0 0 a u a u h a a u e a u me a u = = = = = = πε π h 1 2 12 2 2 2 2 2 2 2 2 2 2 1 2 2 1 2 2 1 2 2 2 1 2 2 1 2 2 12 1 2 1 2 12 2 2 2 1 1 ( ) ˆ ( ) ( ) ( ) 1 ( ) ( ) 2 1 ˆ r r Z r Z V x y z x y z r x x y y z z r r Z r Z H for He atom = − + + ∂ ∂ + ∂ ∂ + ∂ ∂ + ∂ ∂ + ∂ ∂ + ∂ ∂ ∇ = = − + − + − = − ∇ + ∇ − + + i i i j ij i i r r Z H for many electron atoms 1 2 1 ˆ 2 = − ∑ ∇ −∑ +∑ ∑ − is omitted r if ij 1 i i i i r Z H = − ∑ ∇ −∑2 2 1 ˆ = + + + ⋅⋅⋅ = ∑ Ψ = ⋅ ⋅ ⋅⋅⋅ = Π = i i i i n E E1 E2 E3 E 1 ψ1 ψ 2 ψ 3 ψ ψ