正在加载图片...
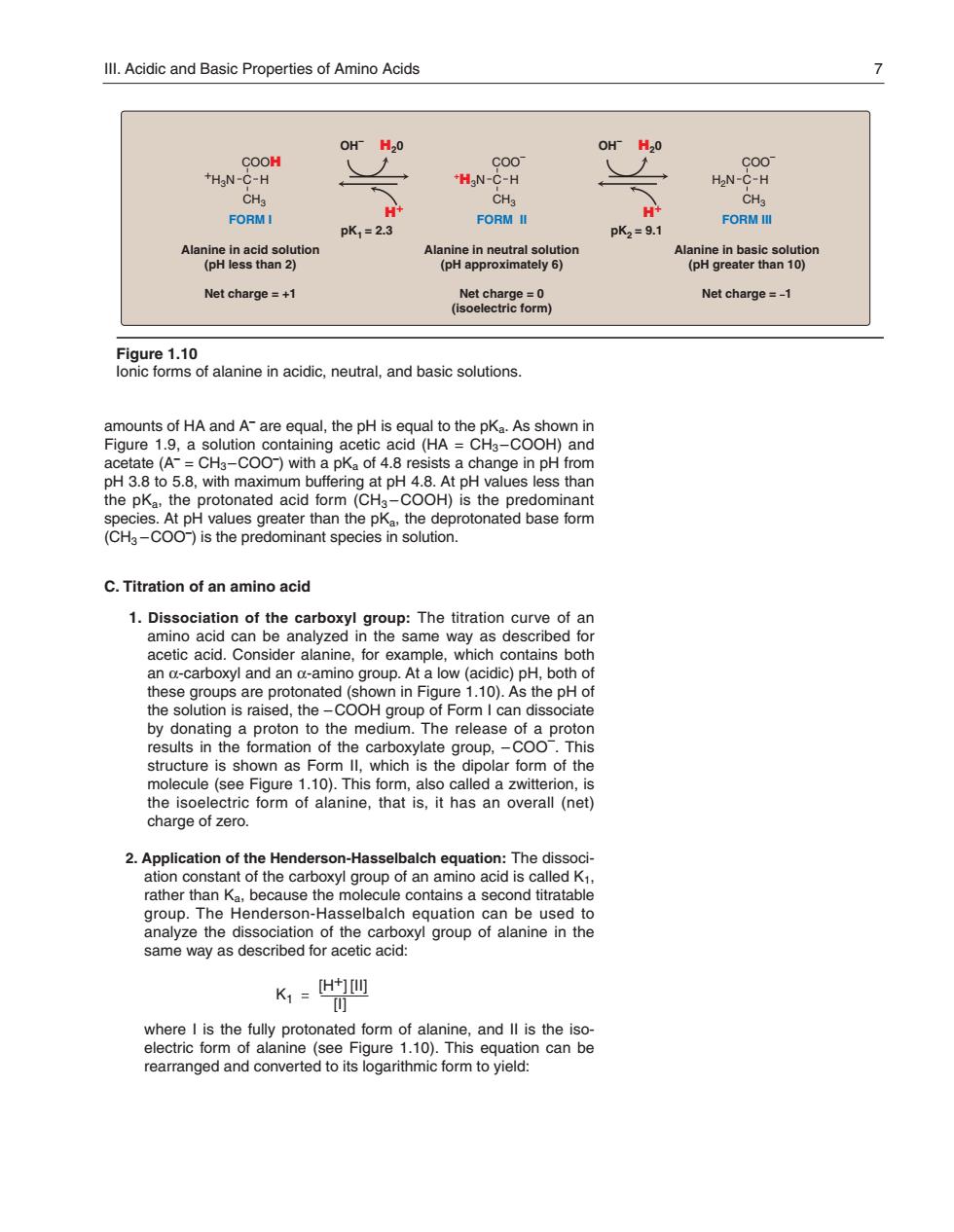
Ill.Acidic and Basic Properties of Amino Acids OH H2O OH H2O THN-COOH HN-OO HN-COO CH. FORMI pK1=2.3 FORM II pK2=9.1 FORM Net charge =+1 Net charge=-1 BaCl8i0anenaidc.erhnalandbassotos iu aC)nd g a to5.8.with maximum buffering at pH 4.8.At pH values less than ues greater than the pKa the protonated base form ninant spe C.Titration of an amino acid 1.Dissociation of the carboxyl group:The titration curve of an amino acid can be analyzed in the same way as described for acetic acid. nsider alanine,for exa contains bo these an ups are protonated (shown in Figure 1.10).As the the solution is raised,the-COOH group of Form I can dissociate lar fo n of the molecule (see Figure 1.10).This form,also called a zwitterion,is h9g8exacomoianethtathasancwealtno创 2.Application of the Henderson-Hasselbalch equation:The dissoci- ation constant of the carboxyl group of an amino acid is called K1. use the e conta is a se d titra same way as described for acetic acid: K,=H四 7ofalhnpot see Fio rearranged and converted to its logarithmic form to yield:amounts of HA and A– are equal, the pH is equal to the pKa. As shown in Figure 1.9, a solution containing acetic acid (HA = CH3 –COOH) and acetate (A– = CH3 –COO– ) with a pKa of 4.8 resists a change in pH from pH 3.8 to 5.8, with maximum buffering at pH 4.8. At pH values less than the pKa, the protonated acid form (CH3 – COOH) is the predominant species. At pH values greater than the pKa, the deprotonated base form (CH3 – COO– ) is the predominant species in solution. C. Titration of an amino acid 1. Dissociation of the carboxyl group: The titration curve of an amino acid can be analyzed in the same way as described for acetic acid. Consider alanine, for example, which contains both an α-carboxyl and an α-amino group. At a low (acidic) pH, both of these groups are proton ated (shown in Figure 1.10). As the pH of the solution is raised, the – COOH group of Form I can dissociate by donating a proton to the medium. The release of a proton results in the formation of the carboxylate group, – COO– . This structure is shown as Form II, which is the dipolar form of the molecule (see Figure 1.10). This form, also called a zwitterion, is the isoelectric form of alanine, that is, it has an overall (net) charge of zero. 2. Application of the Henderson-Hasselbalch equation: The dissociation constant of the carboxyl group of an amino acid is called K1, rather than Ka, because the molecule contains a second titratable group. The Henderson-Hasselbalch equation can be used to analyze the dissociation of the carboxyl group of alanine in the same way as described for acetic acid: where I is the fully protonated form of alanine, and II is the isoelectric form of alanine (see Figure 1.10). This equation can be re arranged and converted to its logarithmic form to yield: III. Acidic and Basic Properties of Amino Acids 7 Figure 1.10 Ionic forms of alanine in acidic, neutral, and basic solutions. COOH FORM I Alanine in acid solution (pH less than 2) Net charge = +1 CH3 C +H3N H COO– FORM II Alanine in neutral solution (pH approximately 6) Net charge = 0 (isoelectric form) CH3 C +H3N H COO– FORM III Alanine in basic solution (pH greater than 10) Net charge = –1 CH3 H2N C H OH H20 – H+ OH H20 – H+ pK1 = 2.3 pK2 = 9.1 K1 [II] [I] [H+] 168397_P001-012.qxd7.0:02 Protein structure 5-20-04 2010.4.4 9:45 AM Page 7