正在加载图片...
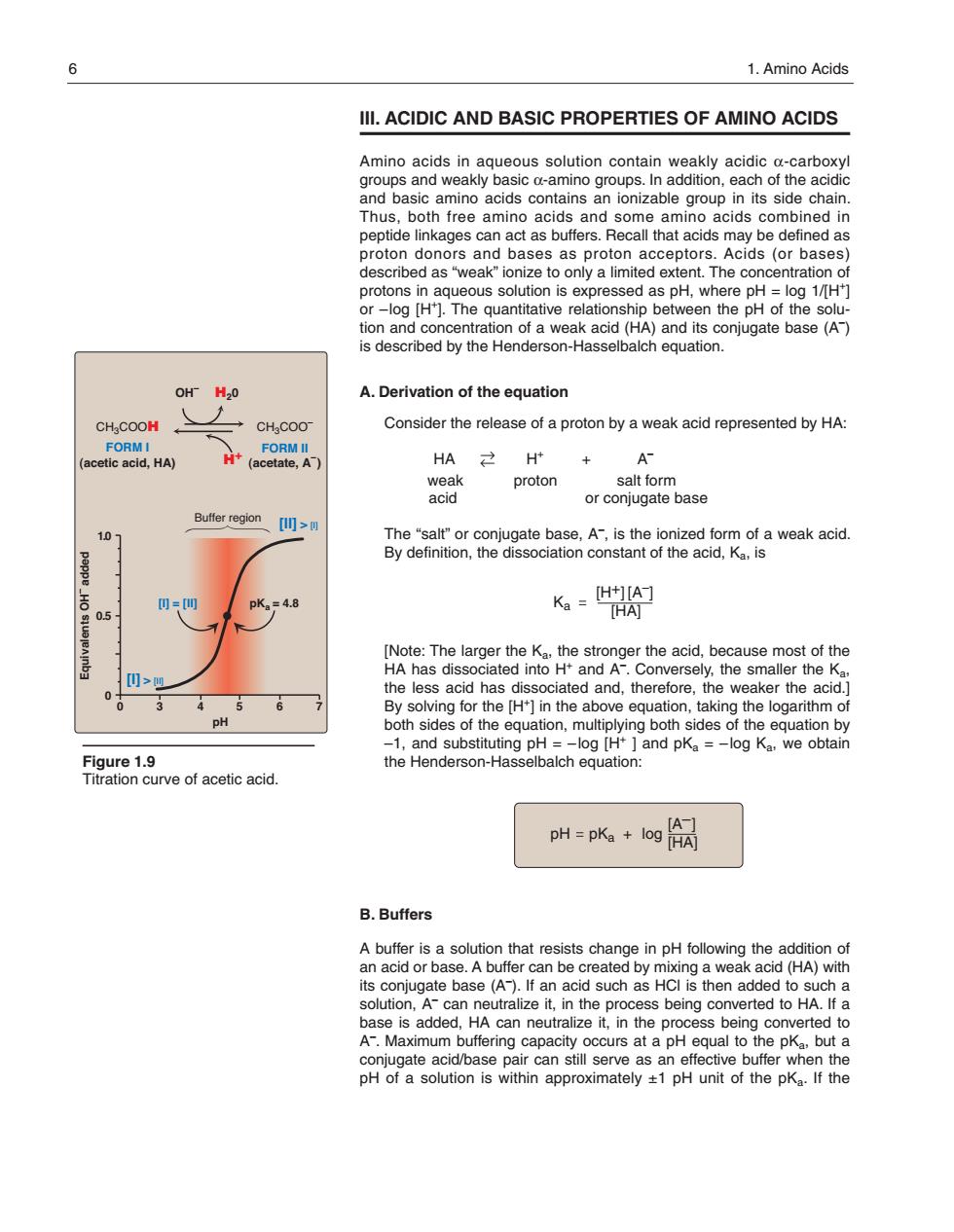
6 1.Amino Acids IlI.ACIDIC AND BASIC PROPERTIES OF AMINO ACIDS 8ead8on28eaoio08asansaaaeaea8te and basic amino acids contains an ionizable group in its side chain acids combine proton donors and bases as proton acceptors.Acids (or bases) described as'"weak"ionize to only a limited extent.The concentration of 8rotogsnaaueoussol ion is expressed as pH.wh ere pr tion and concen aonota8eakacaHanasc0ngaeba9e04 is described by the Henderson-Hasselbalch equation. OH H20 A.Derivation of the equation CH.COOM Consider the release of a proton by a weak acid represented by HA: acetfCcia.Ha t(acetate,A + proton salt form or conjugate base Buffer region 10 Ka =t] 05 06 o the pH both sides of the equation,multiplying both sides of the equation by Figure 1.9 h64easn688agapk-ogKoowa urve of acetic acid. pH=k+6网阁 B.Buffers A buffer is a solutio with solution,A Acan neutralize it,in the process being converted to HA.If a can neutra I, hut conjugate acid base pair can still serve as an effective buffer when the pH of a solution is within approximately +1 pH unit of the pKa.If the III. ACIDIC AND BASIC PROPERTIES OF AMINO ACIDS Amino acids in aqueous solution contain weakly acidic α-carboxyl groups and weakly basic α-amino groups. In addition, each of the acidic and basic amino acids contains an ionizable group in its side chain. Thus, both free amino acids and some amino acids combined in peptide linkages can act as buffers. Recall that acids may be defined as proton donors and bases as proton acceptors. Acids (or bases) described as “weak” ionize to only a limited extent. The concentration of protons in aqueous solution is expressed as pH, where pH = log 1/[H+] or –log [H+]. The quantitative relationship between the pH of the solution and concentration of a weak acid (HA) and its con jugate base (A– ) is described by the Henderson-Hasselbalch equation. A. Derivation of the equation Consider the release of a proton by a weak acid represented by HA: HA →← H+ + A– weak proton salt form acid or conjugate base The “salt” or conjugate base, A– , is the ionized form of a weak acid. By definition, the dissociation constant of the acid, Ka, is [Note: The larger the Ka, the stronger the acid, because most of the HA has dissociated into H+ and A– . Conversely, the smaller the Ka, the less acid has dissociated and, therefore, the weaker the acid.] By solving for the [H+] in the above equation, taking the logarithm of both sides of the equation, multiplying both sides of the equation by –1, and substituting pH = –log [H+ ] and pKa = –log Ka, we obtain the Henderson-Hasselbalch equation: B. Buffers A buffer is a solution that resists change in pH following the addition of an acid or base. A buffer can be created by mixing a weak acid (HA) with its conjugate base (A– ). If an acid such as HCl is then added to such a solution, A– can neutralize it, in the process being converted to HA. If a base is added, HA can neutralize it, in the process being converted to A– . Maximum buffering capacity occurs at a pH equal to the pKa, but a conjugate acid/base pair can still serve as an effective buffer when the pH of a solution is within approximately ±1 pH unit of the pKa. If the 6 1. Amino Acids Figure 1.9 Titration curve of acetic acid. 03 4567 0 0.5 1.0 pH Equivalents OH– added Buffer region CH3COOH CH3COO– H20 FORM I (acetic acid, HA) FORM II (acetate, A– ) [I] = [II] pKa = 4.8 OH– H+ [I] > [II] [II] > [I] pH pKa log [A–] [HA] + Ka [A–] [HA] [H+] 168397_P001-012.qxd7.0:02 Protein structure 5-20-04 2010.4.4 9:45 AM Page 6