正在加载图片...
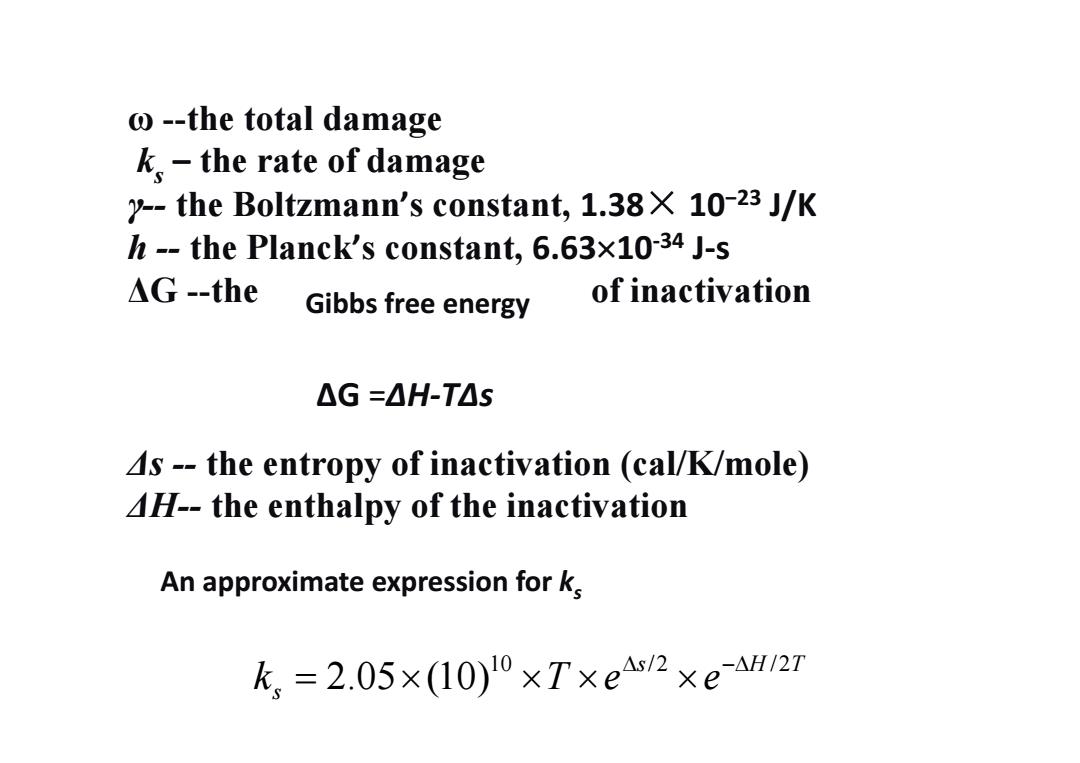
o--the total damage k.the rate of damage y--the Boltzmann's constant,1.38X 10-23 J/K h--the Planck's constant,6.63x10-34 J-s △G-the Gibbs free energy of inactivation △G=△H-T△S 4s--the entropy of inactivation (cal/K/mole) 4H--the enthalpy of the inactivation An approximate expression for k k,=2.05×(10)l0×T×e4s2×eaH/27ω --the total damage ks – the rate of damage γ-- the Boltzmann’s constant, 1.38× 10−23 J/K h -- the Planck’s constant, 6.6310‐34 J‐s ΔG --the of inactivation Δs -- the entropy of inactivation (cal/K/mole) ΔH-- the enthalpy of the inactivation ΔG =ΔH‐TΔs Gibbs free energy 10 /2 /2 2.05 (10) s HT s k Te e An approximate expression for ks