正在加载图片...
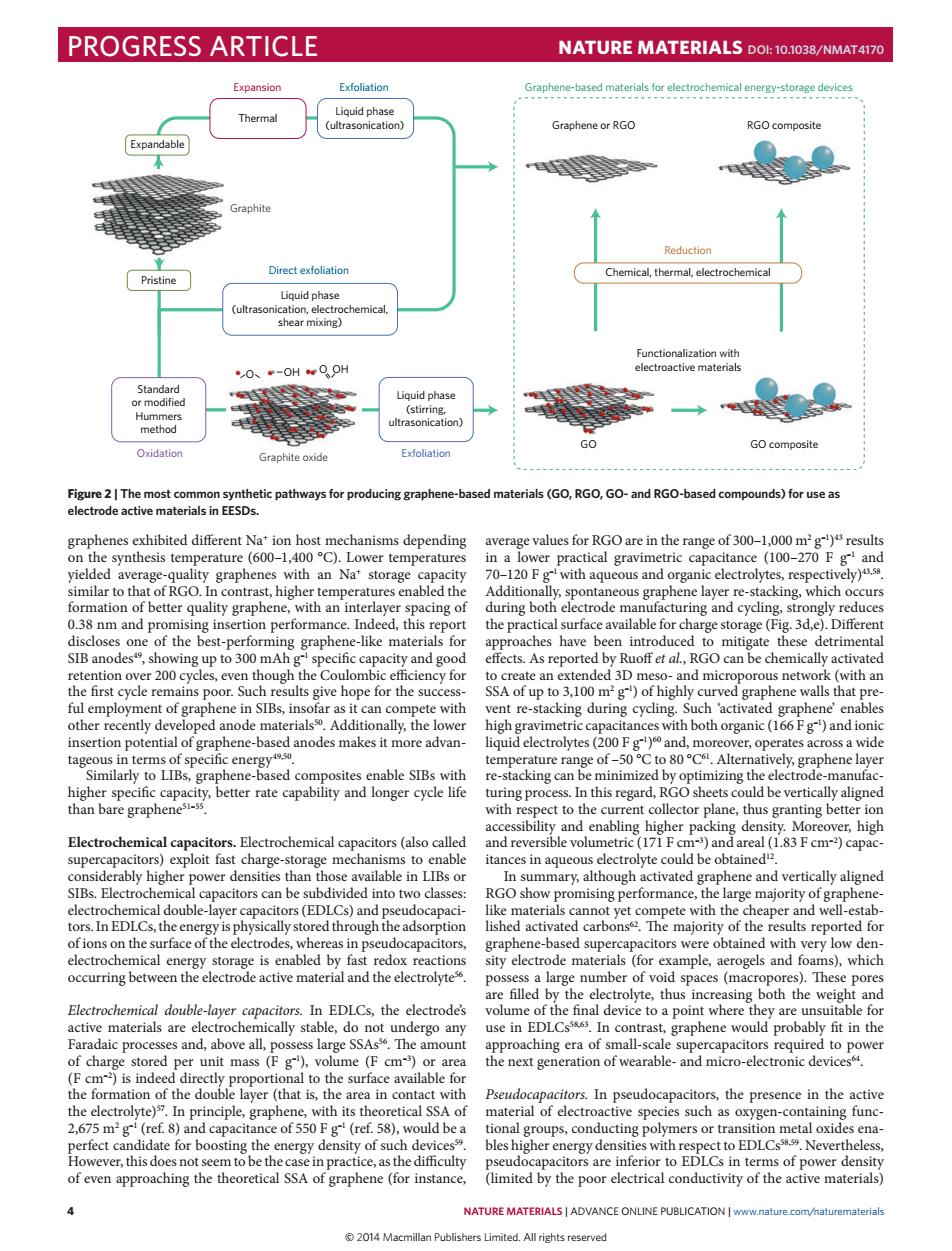
PROGRESS ARTICLE NATURE MATERIALS DOI:10.1038/NMAT4170 Exfoliation ahene-based ma or Pristine hemica shear mixing) 0、-0HQ → GO composite Graphite oxide ways for producing gr (GO,RGO,GO-and RGO-bas nds)for use a ge values for RGO are in the range f0 20F with aqueous and org ing of dompethpor te nodes Similarly capacity,better rate capability and longer cyclet rate ,thus ting better ior Electrochemical ca citors Electrochemical c acitors(also called naete(pan aea(1 acitors can be sube alarge number of void spaces (mac Electrochemical double-ayer electrode's the ey are u and above all. In the presence in the active ops,contctngPonesoetr metaloides s of power dens of even approaching the theoretical SSA of graphene (for instance limited by the poor electrical conductivity of the active materials NATURE MATERIALS|ADVANCE ONLINE PUBLICATIONwwnaturecom/turemaeri 2014 Macmillan Publishers Limited All rights reserved 4 NATURE MATERIALS | ADVANCE ONLINE PUBLICATION | www.nature.com/naturematerials graphenes exhibited different Na+ ion host mechanisms depending on the synthesis temperature (600–1,400 °C). Lower temperatures yielded average-quality graphenes with an Na+ storage capacity similar to that of RGO. In contrast, higher temperatures enabled the formation of better quality graphene, with an interlayer spacing of 0.38 nm and promising insertion performance. Indeed, this report discloses one of the best-performing graphene-like materials for SIB anodes49, showing up to 300 mAh g–1 specific capacity and good retention over 200 cycles, even though the Coulombic efficiency for the first cycle remains poor. Such results give hope for the successful employment of graphene in SIBs, insofar as it can compete with other recently developed anode materials50. Additionally, the lower insertion potential of graphene-based anodes makes it more advantageous in terms of specific energy49,50. Similarly to LIBs, graphene-based composites enable SIBs with higher specific capacity, better rate capability and longer cycle life than bare graphene51–55. Electrochemical capacitors. Electrochemical capacitors (also called supercapacitors) exploit fast charge-storage mechanisms to enable considerably higher power densities than those available in LIBs or SIBs. Electrochemical capacitors can be subdivided into two classes: electrochemical double-layer capacitors (EDLCs) and pseudocapacitors. In EDLCs, the energy is physically stored through the adsorption of ions on the surface of the electrodes, whereas in pseudocapacitors, electrochemical energy storage is enabled by fast redox reactions occurring between the electrode active material and the electrolyte56. Electrochemical double-layer capacitors. In EDLCs, the electrode’s active materials are electrochemically stable, do not undergo any Faradaic processes and, above all, possess large SSAs56. The amount of charge stored per unit mass (F g–1), volume (F cm–3) or area (F cm–2) is indeed directly proportional to the surface available for the formation of the double layer (that is, the area in contact with the electrolyte)57. In principle, graphene, with its theoretical SSA of 2,675 m2 g–1 (ref. 8) and capacitance of 550 F g–1 (ref. 58), would be a perfect candidate for boosting the energy density of such devices59. However, this does not seem to be the case in practice, as the difficulty of even approaching the theoretical SSA of graphene (for instance, average values for RGO are in the range of 300–1,000 m2 g–1)43 results in a lower practical gravimetric capacitance (100–270 F g–1 and 70–120 F g–1 with aqueous and organic electrolytes, respectively)43,58. Additionally, spontaneous graphene layer re-stacking, which occurs during both electrode manufacturing and cycling, strongly reduces the practical surface available for charge storage (Fig. 3d,e). Different approaches have been introduced to mitigate these detrimental effects. As reported by Ruoff et al., RGO can be chemically activated to create an extended 3D meso- and microporous network (with an SSA of up to 3,100 m2 g–1) of highly curved graphene walls that prevent re-stacking during cycling. Such ‘activated graphene’ enables high gravimetric capacitances with both organic (166 F g–1) and ionic liquid electrolytes (200 F g–1)60 and, moreover, operates across a wide temperature range of –50 °C to 80 °C61. Alternatively, graphene layer re-stacking can be minimized by optimizing the electrode-manufacturing process. In this regard, RGO sheets could be vertically aligned with respect to the current collector plane, thus granting better ion accessibility and enabling higher packing density. Moreover, high and reversible volumetric (171 F cm–3) and areal (1.83 F cm–2) capacitances in aqueous electrolyte could be obtained12. In summary, although activated graphene and vertically aligned RGO show promising performance, the large majority of graphenelike materials cannot yet compete with the cheaper and well-established activated carbons62. The majority of the results reported for graphene-based supercapacitors were obtained with very low density electrode materials (for example, aerogels and foams), which possess a large number of void spaces (macropores). These pores are filled by the electrolyte, thus increasing both the weight and volume of the final device to a point where they are unsuitable for use in EDLCs58,63. In contrast, graphene would probably fit in the approaching era of small-scale supercapacitors required to power the next generation of wearable- and micro-electronic devices64. Pseudocapacitors. In pseudocapacitors, the presence in the active material of electroactive species such as oxygen-containing functional groups, conducting polymers or transition metal oxides enables higher energy densities with respect to EDLCs58,59. Nevertheless, pseudocapacitors are inferior to EDLCs in terms of power density (limited by the poor electrical conductivity of the active materials) Graphite Expandable Direct exfoliation Liquid phase (ultrasonication, electrochemical, shear mixing) Liquid phase (stirring, ultrasonication) Gr Exfoliation aphite oxide GO GO composite O Exfoliation Liquid phase (ultrasonication) Expansion Thermal Standard or modified Hummers method Oxidation Graphene-based materials for electrochemical energy-storage devices Graphene or RGO RGO composite Functionalization with electroactive materials Pristine Chemical, thermal, electrochemical Reduction OH O OH Figure 2 | The most common synthetic pathways for producing graphene-based materials (GO, RGO, GO- and RGO-based compounds) for use as electrode active materials in EESDs. PROGRESS ARTICLE NATURE MATERIALS DOI: 10.1038/NMAT4170 © 2014 Macmillan Publishers Limited. All rights reserved