正在加载图片...
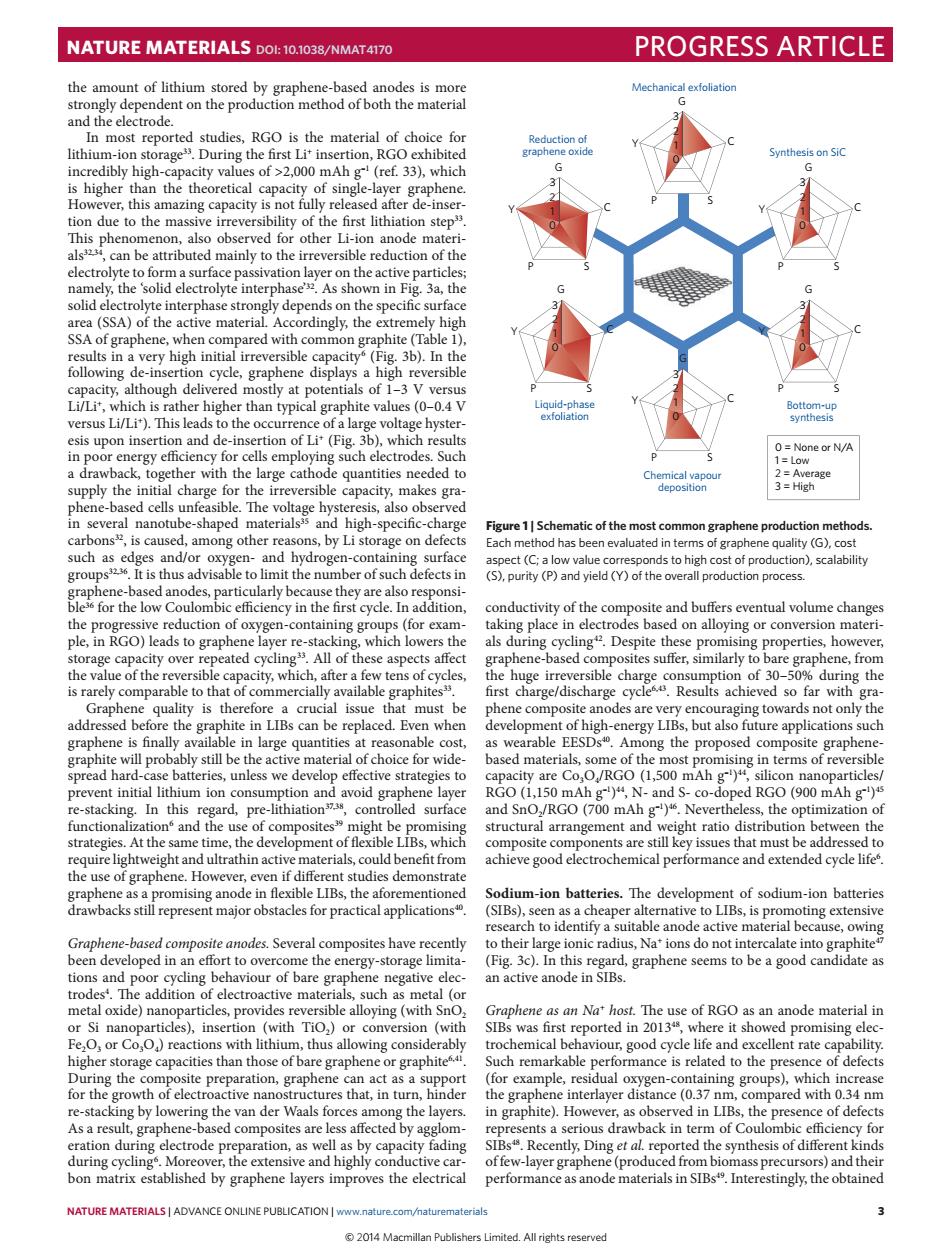
NATURE MATERIALS DOL:10.1038/NMAT4170 PROGRESS ARTICLE the a nt of lithium Mechanical extiotiatio depend In most re rted studies,RGO is the material of choice for (ref.33).which is higher thisn the lity of the first lithiation ste ompared with commo ohite (Tab Liud-gha voltage hvs (od None or N/ quan .by Li stora atic of the most com of the c ite and buffers ntual volume ch nleoc-con ing place in capa ity over affec h彩eoRutiohCf00%rcdwrm8th I issue mus b robe the graphene is finall ong the p ed comr ppte graphen preve ed RGO(900 mA In thi N.co-o tructu atio distribu ween th Sodium-ion batteries The develo ent of sodium-ion hatterie draw major obstacles for practical applications' SIBs ch ode ernati Graphene-based comp des.Several composites have ently ions do not our of bare e a Rood ca meta icla asNat host The use of rgo as an anode material ir Fe.O. was h reported in 2013 ere it sho higher sto raphite h rem rkable p ce of defect of e prese Esordcfet ctrode prep ation,as well fading ng et ed the synthesis of diffe d bon matrix established by graphene layers improves the electrical NATURE MATERIALS LADVANCE ONLINE PUBLICATIONIWWWDabur 3NATURE MATERIALS | ADVANCE ONLINE PUBLICATION | www.nature.com/naturematerials 3 the amount of lithium stored by graphene-based anodes is more strongly dependent on the production method of both the material and the electrode. In most reported studies, RGO is the material of choice for lithium-ion storage33. During the first Li+ insertion, RGO exhibited incredibly high-capacity values of >2,000 mAh g–1 (ref. 33), which is higher than the theoretical capacity of single-layer graphene. However, this amazing capacity is not fully released after de-insertion due to the massive irreversibility of the first lithiation step33. This phenomenon, also observed for other Li-ion anode materials32,34, can be attributed mainly to the irreversible reduction of the electrolyte to form a surface passivation layer on the active particles; namely, the ‘solid electrolyte interphase’32. As shown in Fig. 3a, the solid electrolyte interphase strongly depends on the specific surface area (SSA) of the active material. Accordingly, the extremely high SSA of graphene, when compared with common graphite (Table 1), results in a very high initial irreversible capacity6 (Fig. 3b). In the following de-insertion cycle, graphene displays a high reversible capacity, although delivered mostly at potentials of 1–3 V versus Li/Li+, which is rather higher than typical graphite values (0–0.4 V versus Li/Li+). This leads to the occurrence of a large voltage hysteresis upon insertion and de-insertion of Li+ (Fig. 3b), which results in poor energy efficiency for cells employing such electrodes. Such a drawback, together with the large cathode quantities needed to supply the initial charge for the irreversible capacity, makes graphene-based cells unfeasible. The voltage hysteresis, also observed in several nanotube-shaped materials35 and high-specific-charge carbons32, is caused, among other reasons, by Li storage on defects such as edges and/or oxygen- and hydrogen-containing surface groups32,36. It is thus advisable to limit the number of such defects in graphene-based anodes, particularly because they are also responsible36 for the low Coulombic efficiency in the first cycle. In addition, the progressive reduction of oxygen-containing groups (for example, in RGO) leads to graphene layer re-stacking, which lowers the storage capacity over repeated cycling33. All of these aspects affect the value of the reversible capacity, which, after a few tens of cycles, is rarely comparable to that of commercially available graphites33. Graphene quality is therefore a crucial issue that must be addressed before the graphite in LIBs can be replaced. Even when graphene is finally available in large quantities at reasonable cost, graphite will probably still be the active material of choice for widespread hard-case batteries, unless we develop effective strategies to prevent initial lithium ion consumption and avoid graphene layer re-stacking. In this regard, pre-lithiation37,38, controlled surface functionalization6 and the use of composites39 might be promising strategies. At the same time, the development of flexible LIBs, which require lightweight and ultrathin active materials, could benefit from the use of graphene. However, even if different studies demonstrate graphene as a promising anode in flexible LIBs, the aforementioned drawbacks still represent major obstacles for practical applications40. Graphene-based composite anodes. Several composites have recently been developed in an effort to overcome the energy-storage limitations and poor cycling behaviour of bare graphene negative electrodes4 . The addition of electroactive materials, such as metal (or metal oxide) nanoparticles, provides reversible alloying (with SnO2 or Si nanoparticles), insertion (with TiO2) or conversion (with Fe2O3 or Co3O4) reactions with lithium, thus allowing considerably higher storage capacities than those of bare graphene or graphite6,41. During the composite preparation, graphene can act as a support for the growth of electroactive nanostructures that, in turn, hinder re-stacking by lowering the van der Waals forces among the layers. As a result, graphene-based composites are less affected by agglomeration during electrode preparation, as well as by capacity fading during cycling6 . Moreover, the extensive and highly conductive carbon matrix established by graphene layers improves the electrical conductivity of the composite and buffers eventual volume changes taking place in electrodes based on alloying or conversion materials during cycling42. Despite these promising properties, however, graphene-based composites suffer, similarly to bare graphene, from the huge irreversible charge consumption of 30–50% during the first charge/discharge cycle6,43. Results achieved so far with graphene composite anodes are very encouraging towards not only the development of high-energy LIBs, but also future applications such as wearable EESDs40. Among the proposed composite graphenebased materials, some of the most promising in terms of reversible capacity are Co3O4/RGO (1,500 mAh g–1)44, silicon nanoparticles/ RGO (1,150 mAh g–1)44, N- and S- co-doped RGO (900 mAh g–1)45 and SnO2/RGO (700 mAh g–1)46. Nevertheless, the optimization of structural arrangement and weight ratio distribution between the composite components are still key issues that must be addressed to achieve good electrochemical performance and extended cycle life6 . Sodium-ion batteries. The development of sodium-ion batteries (SIBs), seen as a cheaper alternative to LIBs, is promoting extensive research to identify a suitable anode active material because, owing to their large ionic radius, Na+ ions do not intercalate into graphite47 (Fig. 3c). In this regard, graphene seems to be a good candidate as an active anode in SIBs. Graphene as an Na+ host. The use of RGO as an anode material in SIBs was first reported in 201348, where it showed promising electrochemical behaviour, good cycle life and excellent rate capability. Such remarkable performance is related to the presence of defects (for example, residual oxygen-containing groups), which increase the graphene interlayer distance (0.37 nm, compared with 0.34 nm in graphite). However, as observed in LIBs, the presence of defects represents a serious drawback in term of Coulombic efficiency for SIBs48. Recently, Ding et al. reported the synthesis of different kinds of few-layer graphene (produced from biomass precursors) and their performance as anode materials in SIBs49. Interestingly, the obtained Mechanical exfoliation Chemical vapour deposition Reduction of graphene oxide Synthesis on SiC 0 = None or N/A 1 = Low 2 = Average 3 = High Liquid-phase exfoliation Bottom-up synthesis P S C G Y P S C G Y P S C G Y P S C G Y P S C G Y P S C G Y 0 1 2 3 0 1 2 3 0 1 2 3 0 1 2 3 0 1 2 3 0 1 2 3 Figure 1 | Schematic of the most common graphene production methods. Each method has been evaluated in terms of graphene quality (G), cost aspect (C; a low value corresponds to high cost of production), scalability (S), purity (P) and yield (Y) of the overall production process. NATURE MATERIALS DOI: 10.1038/NMAT4170 PROGRESS ARTICLE © 2014 Macmillan Publishers Limited. All rights reserved