正在加载图片...
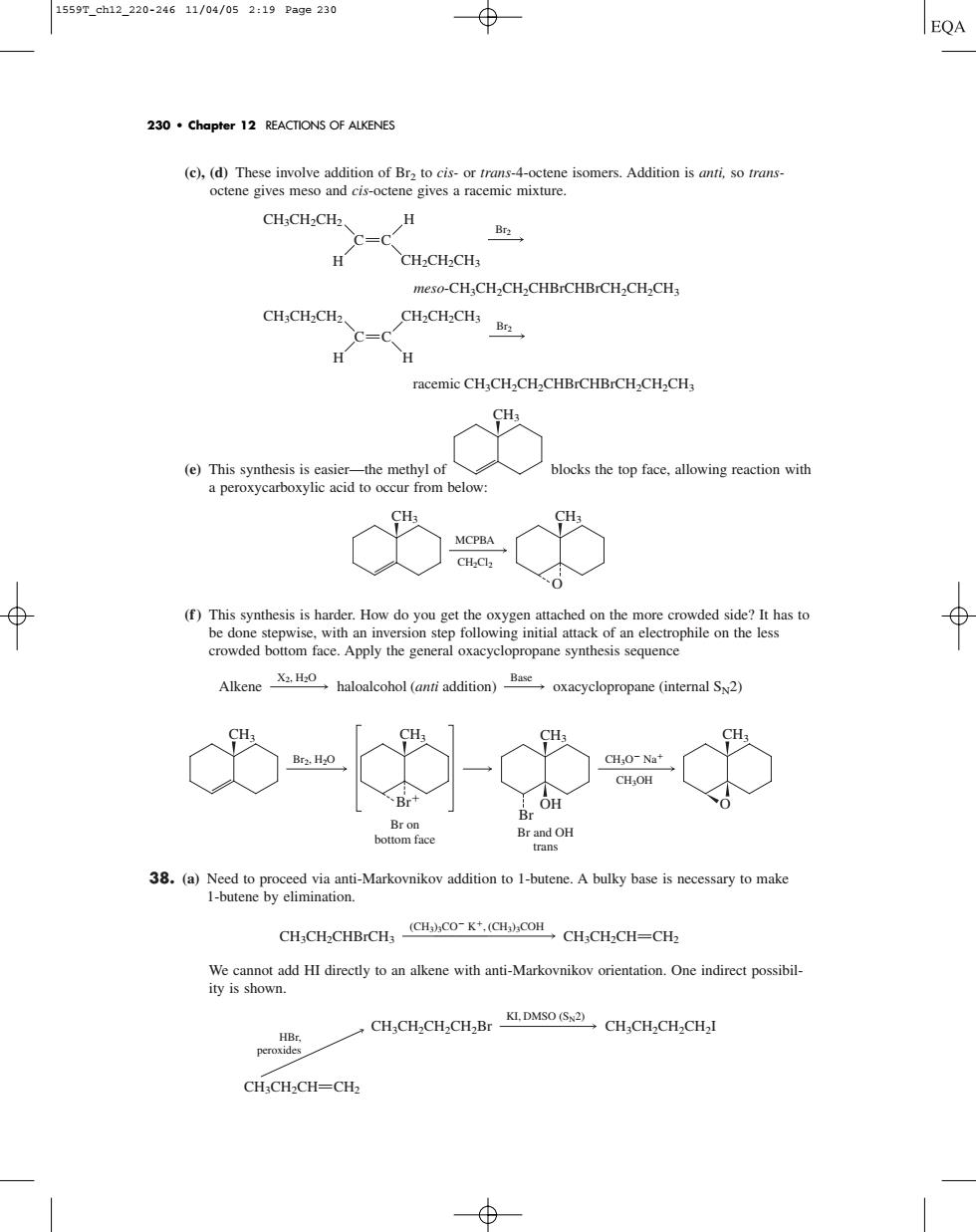
1559T_ch12_220-24611/04/052:19Page230 EQA 230.Chapter 12 REACTIONS OF ALKENES ne isomers.Addition is anti,so trans CH:CH-CH c=c CH-CH-CH: CH:CH2CH2 CH2CH2CHs H racemic CH.CH2CH2CHBrCHBrCH.CH.CH blocks the top face.allowing reaction with occur from (f)This synthesis is harder.How do you get the oxygen attached on the more crowded bottom face.Apply the general OH OH 38.(a)Need to proceed via anti-Markovnikov addition to I-butene.A bulky base is necessary to make CH,CH-CHBCH,CH.CH-CH-CH ediretan with nMakoniaioendiret possib CH-CH-CH-CH-Br KLDMSO (S2)CH-CH-CH-CHal CHCH2CH-CH2 (c), (d) These involve addition of Br2 to cis- or trans-4-octene isomers. Addition is anti, so transoctene gives meso and cis-octene gives a racemic mixture. (e) This synthesis is easier—the methyl of blocks the top face, allowing reaction with a peroxycarboxylic acid to occur from below: (f ) This synthesis is harder. How do you get the oxygen attached on the more crowded side? It has to be done stepwise, with an inversion step following initial attack of an electrophile on the less crowded bottom face. Apply the general oxacyclopropane synthesis sequence 38. (a) Need to proceed via anti-Markovnikov addition to 1-butene. A bulky base is necessary to make 1-butene by elimination. We cannot add HI directly to an alkene with anti-Markovnikov orientation. One indirect possibility is shown. CH3CH2CH2CH2Br KI, DMSO (SN2) CH3CH2CH2CH2I CH3CH2CH CH2 HBr, peroxides (CH3)3CO K, (CH3)3COH CH3CH2CHBrCH3 CH3CH2CH CH2 CH3 Br on bottom face Br2, H2O CH3 Br CH3O Na CH3OH CH3 O Br and OH trans CH3 Br OH Alkene haloalcohol (anti addition) oxacyclopropane (internal SN2) X2, H2O Base CH3 CH3 O MCPBA CH2Cl2 CH3 Br2 CH2CH2CH3 meso-CH3CH2CH2CHBrCHBrCH2CH2CH3 CH3CH2CH2 H H C C racemic CH3CH2CH2CHBrCHBrCH2CH2CH3 Br2 CH3CH2CH2 CH2CH2CH3 H H C C 230 • Chapter 12 REACTIONS OF ALKENES 1559T_ch12_220-246 11/04/05 2:19 Page 230��