正在加载图片...
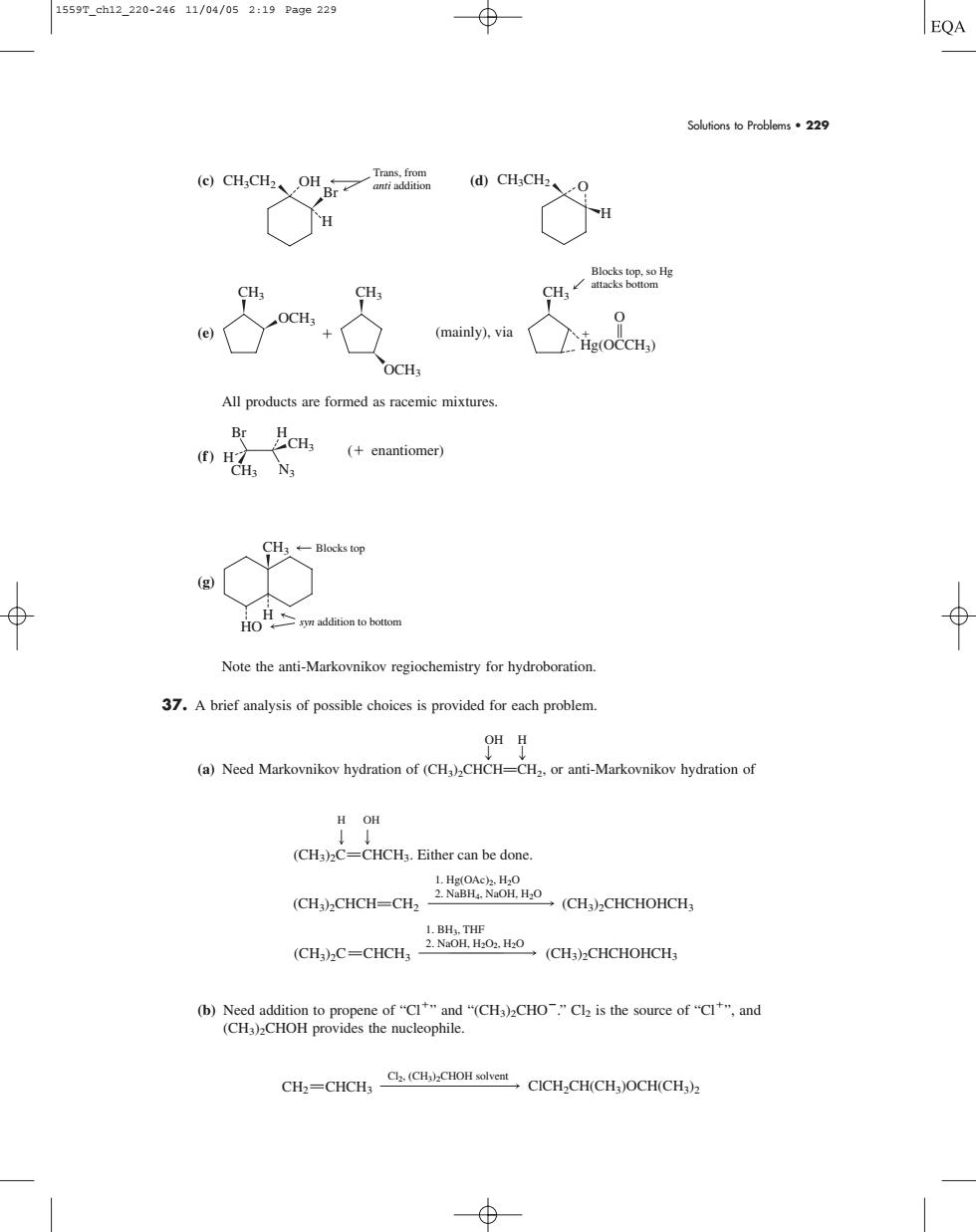
15597.eh12.220-24611/0a/052:19Page229 EQA Solutions o Problems229 dCH,cH0 H CH o. 0 (mainly),via g(OCCH) All products are formed as racemic mixtures (+enantiomer) g 风 Note the anti-Markovnikov regiochemistry for hydroboration. 37.A brief analysis of possible choices is provided for each problem oH月 (a)Need Markovnikov hydration of (CH)CHCH-CH or anti-Markovnikov hydration o H OH (CH3)2C=CHCH3.Either can be done. GG-0 CHC-CCCUCCHOC CHCHCH,CICH,CHCHOCHCH)Solutions to Problems • 229 (c) (d) (e) All products are formed as racemic mixtures. (f ) (g) Note the anti-Markovnikov regiochemistry for hydroboration. 37. A brief analysis of possible choices is provided for each problem. OH H g g (a) Need Markovnikov hydration of (CH3)2CHCHPCH2, or anti-Markovnikov hydration of (b) Need addition to propene of “Cl” and “(CH3)2CHO.” Cl2 is the source of “Cl”, and (CH3)2CHOH provides the nucleophile. Cl2, (CH3)2CHOH solvent CH2 CHCH3 ClCH2CH(CH3)OCH(CH3)2 1. Hg(OAc)2, H2O 2. NaBH4, NaOH, H2O (CH3)2C (CH3)2CHCH (CH3)2CHCHOHCH3 H OH CHCH3. Either can be done. CH2 1. BH3, THF 2. NaOH, H2O2, H2O (CH3)2C CHCH3 (CH3)2CHCHOHCH3 H HO CH3 Blocks top syn addition to bottom CH3 N3 CH3 Br ( enantiomer) H H CH3 OCH3 (mainly), via CH3 CH3 O Blocks top, so Hg attacks bottom Hg(OCCH3) OCH3 CH3CH2 O H CH3CH2 OH Trans, from anti addition Br H 1559T_ch12_220-246 11/04/05 2:19 Page 229�