正在加载图片...
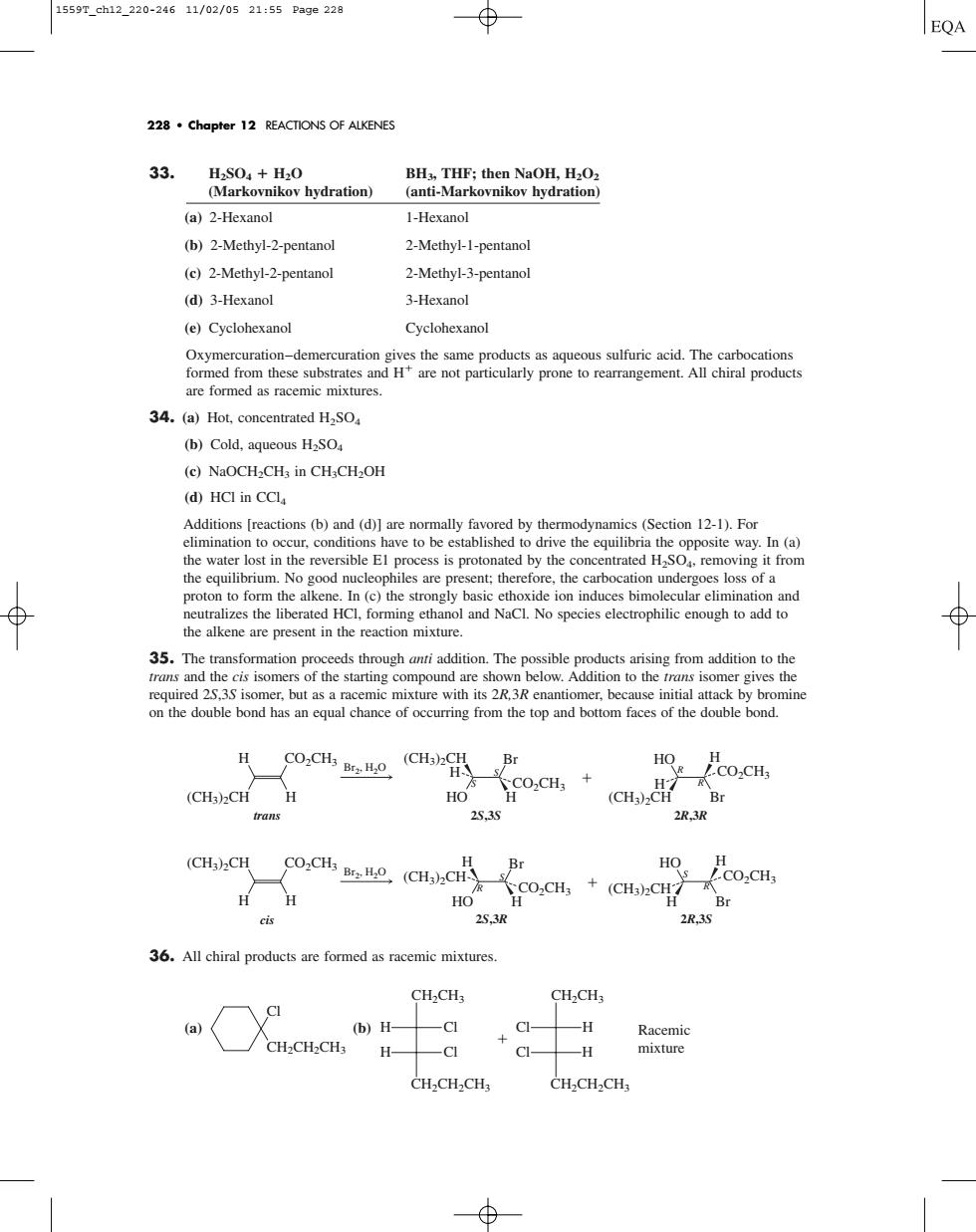
1559T_ch12_220-24611/02/0521:55Pa9e228 EQA 228.Chapter 12 REACTIONS OF ALKENES 33. (a)2-Hexanol 1-Hexanol (b)2-Methy1-2-pentanol 2-Methyl-1-pentanol (c)2-Methyl-2-pentanol 2-Methyl-3-pentanol (d)3-Hexanol 3-Hexanol (e)Cyclohexanol Cyclohexanol Oxymercuration-dem theare not paricularly prone to rearrangememt roducts 34.(a)Hot,concentrated H2SO (b)Cold,aqueous H2SO (c)NaOCH,CH in CHCH,OH (d)HCI in CCl Additions [reactions(b)and(d)]are normally favored by thermodynamics(Section 12-1).For ave to b the No good nucleophiles are present:therefore.the carbocation under goes loss of a on the double bond has an equal chance of occurring from the top and bottom faces of the double bond. H Co.CH,o (CH) H。H O.CHs (CHs)CH H HO CO:CH+ (CHa)CH Br 253S 2R,3R (CH)2CH Co.c,(CH).c H H Co.CH,+(CH).CH Br cis 253 2R3 36.All chiral products are formed as racemic mixtures CH2CH3 CH2CH3 (b)H- -CI CI- -H Racemi H mixture CH-CH-CHs CH.CH2CH 33. H2SO4 H2O BH3, THF; then NaOH, H2O2 (Markovnikov hydration) (anti-Markovnikov hydration) (a) 2-Hexanol 1-Hexanol (b) 2-Methyl-2-pentanol 2-Methyl-1-pentanol (c) 2-Methyl-2-pentanol 2-Methyl-3-pentanol (d) 3-Hexanol 3-Hexanol (e) Cyclohexanol Cyclohexanol Oxymercuration–demercuration gives the same products as aqueous sulfuric acid. The carbocations formed from these substrates and H are not particularly prone to rearrangement. All chiral products are formed as racemic mixtures. 34. (a) Hot, concentrated H2SO4 (b) Cold, aqueous H2SO4 (c) NaOCH2CH3 in CH3CH2OH (d) HCl in CCl4 Additions [reactions (b) and (d)] are normally favored by thermodynamics (Section 12-1). For elimination to occur, conditions have to be established to drive the equilibria the opposite way. In (a) the water lost in the reversible E1 process is protonated by the concentrated H2SO4, removing it from the equilibrium. No good nucleophiles are present; therefore, the carbocation undergoes loss of a proton to form the alkene. In (c) the strongly basic ethoxide ion induces bimolecular elimination and neutralizes the liberated HCl, forming ethanol and NaCl. No species electrophilic enough to add to the alkene are present in the reaction mixture. 35. The transformation proceeds through anti addition. The possible products arising from addition to the trans and the cis isomers of the starting compound are shown below. Addition to the trans isomer gives the required 2S,3S isomer, but as a racemic mixture with its 2R,3R enantiomer, because initial attack by bromine on the double bond has an equal chance of occurring from the top and bottom faces of the double bond. 36. All chiral products are formed as racemic mixtures. (a) (b) CH2CH2CH3 CH2CH3 Cl H Cl H Racemic mixture CH2CH2CH3 CH2CH3 Cl Cl H H CH2CH2CH3 Cl HO H CO2CH3 CO2CH3 (CH3)2CH (CH3)2CH H H H S S Br (CH3)2CH CO2CH3 R R Br HO H H H trans 2S,3S 2R,3R Br2, H2O HO H CO2CH3 (CH3)2CH (CH3)2CH CO2CH3 H H H S R Br (CH3)2CH CO2CH3 S R Br HO H cis 2S,3R 2R,3S Br2, H2O 228 • Chapter 12 REACTIONS OF ALKENES 1559T_ch12_220-246 11/02/05 21:55 Page 228