正在加载图片...
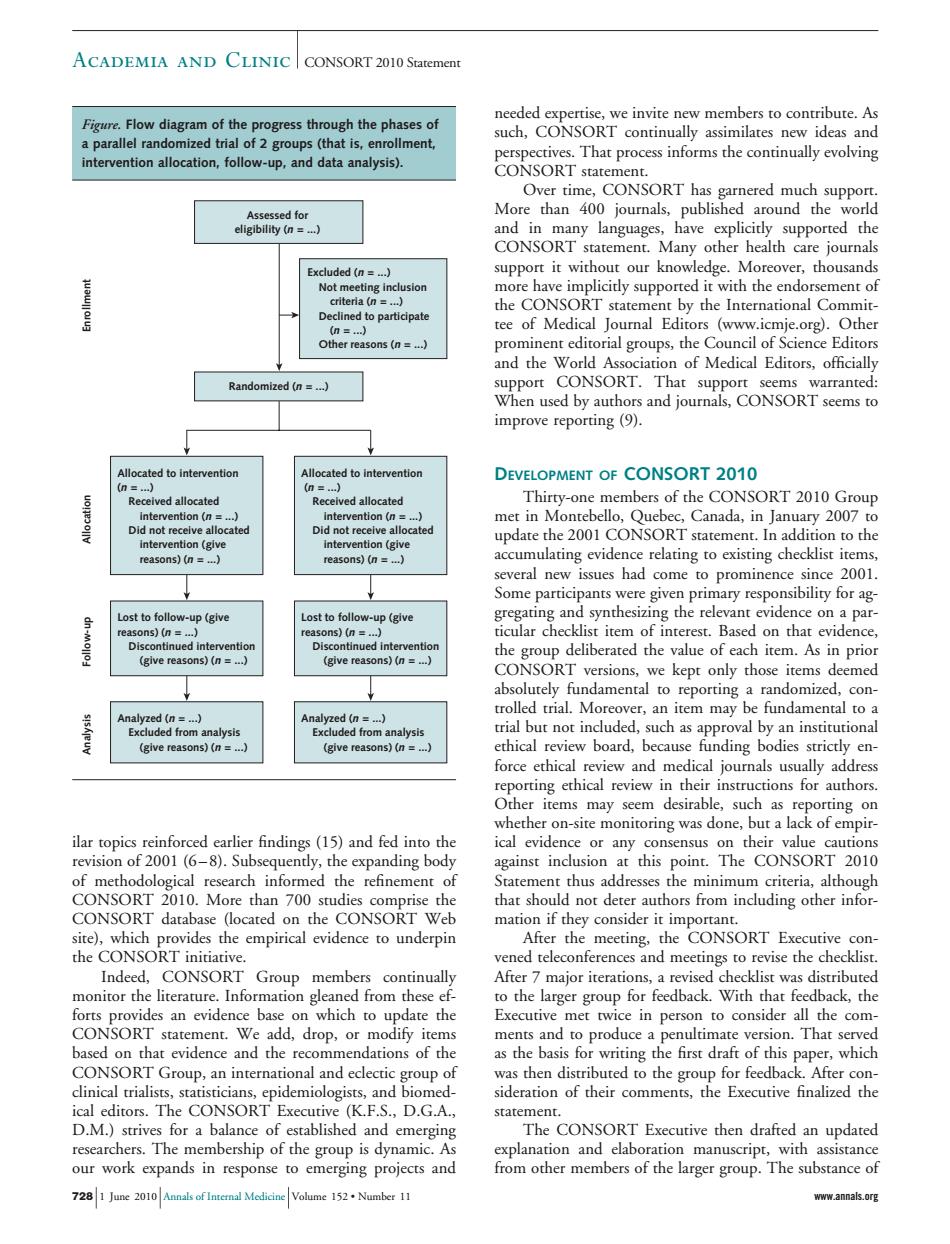
ACADEMIA AND CLINIC CONSORT 2010 Starement ure.A That p intervention allocation,follow p and data analysis) CONSORT statement. CONoRTbniagnerdghpo More than 400 journals,publ around the support it without our knowledge.Morcover,thousands more have implicitly supported it with the endorsement of o participate tee of nthWorlsci f Medical rf Randomized (n=) ed to in DEVELOPMENT OF CONSORT 2010 ed alla ed allocated Thirty-one members of the CONSORT 2010 Group ention (n=. te Canada,in January 200 easons)(n=) several new issues had come Some participants were given primary responsibility for ag Lost to follow- ons)(= give rea ons)(n) (give reasons)n) uc hlit irm of ine (give reasons)(n (give reasons) thica msm or any of methodolo al re t o t thus addres inim that should not deter authors from including other infor te mecting. ORT E Indeed.CONSORT Gro monitor the literature.Information g aned from thes to the larger group for feedback.With that feedback,the Executive met twice in person to consider all the com nts an n.That ser the b nrst ara :L sideration of their comments.the Executive finalized the ical editors.The CONSORT Executive (K.F.S.D.G.A. statement DheThp of thego The CONSORT Executive then drafted an updated expa emerging projects larger group. nal Medicine Volume 152.Number 11 ilar topics reinforced earlier findings (15) and fed into the revision of 2001 (6 – 8). Subsequently, the expanding body of methodological research informed the refinement of CONSORT 2010. More than 700 studies comprise the CONSORT database (located on the CONSORT Web site), which provides the empirical evidence to underpin the CONSORT initiative. Indeed, CONSORT Group members continually monitor the literature. Information gleaned from these efforts provides an evidence base on which to update the CONSORT statement. We add, drop, or modify items based on that evidence and the recommendations of the CONSORT Group, an international and eclectic group of clinical trialists, statisticians, epidemiologists, and biomedical editors. The CONSORT Executive (K.F.S., D.G.A., D.M.) strives for a balance of established and emerging researchers. The membership of the group is dynamic. As our work expands in response to emerging projects and needed expertise, we invite new members to contribute. As such, CONSORT continually assimilates new ideas and perspectives. That process informs the continually evolving CONSORT statement. Over time, CONSORT has garnered much support. More than 400 journals, published around the world and in many languages, have explicitly supported the CONSORT statement. Many other health care journals support it without our knowledge. Moreover, thousands more have implicitly supported it with the endorsement of the CONSORT statement by the International Committee of Medical Journal Editors (www.icmje.org). Other prominent editorial groups, the Council of Science Editors and the World Association of Medical Editors, officially support CONSORT. That support seems warranted: When used by authors and journals, CONSORT seems to improve reporting (9). DEVELOPMENT OF CONSORT 2010 Thirty-one members of the CONSORT 2010 Group met in Montebello, Quebec, Canada, in January 2007 to update the 2001 CONSORT statement. In addition to the accumulating evidence relating to existing checklist items, several new issues had come to prominence since 2001. Some participants were given primary responsibility for aggregating and synthesizing the relevant evidence on a particular checklist item of interest. Based on that evidence, the group deliberated the value of each item. As in prior CONSORT versions, we kept only those items deemed absolutely fundamental to reporting a randomized, controlled trial. Moreover, an item may be fundamental to a trial but not included, such as approval by an institutional ethical review board, because funding bodies strictly enforce ethical review and medical journals usually address reporting ethical review in their instructions for authors. Other items may seem desirable, such as reporting on whether on-site monitoring was done, but a lack of empirical evidence or any consensus on their value cautions against inclusion at this point. The CONSORT 2010 Statement thus addresses the minimum criteria, although that should not deter authors from including other information if they consider it important. After the meeting, the CONSORT Executive convened teleconferences and meetings to revise the checklist. After 7 major iterations, a revised checklist was distributed to the larger group for feedback. With that feedback, the Executive met twice in person to consider all the comments and to produce a penultimate version. That served as the basis for writing the first draft of this paper, which was then distributed to the group for feedback. After consideration of their comments, the Executive finalized the statement. The CONSORT Executive then drafted an updated explanation and elaboration manuscript, with assistance from other members of the larger group. The substance of Figure. Flow diagram of the progress through the phases of a parallel randomized trial of 2 groups (that is, enrollment, intervention allocation, follow-up, and data analysis). Assessed for eligibility (n = ...) Enrollment Allocation Follow-up Randomized (n = ...) Excluded (n = ...) Not meeting inclusion criteria (n = ...) Declined to participate (n = ...) Other reasons (n = ...) Allocated to intervention (n = ...) Received allocated intervention (n = ...) Did not receive allocated intervention (give reasons) (n = ...) Allocated to intervention (n = ...) Received allocated intervention (n = ...) Did not receive allocated intervention (give reasons) (n = ...) Lost to follow-up (give reasons) (n = ...) Discontinued intervention (give reasons) (n = ...) Lost to follow-up (give reasons) (n = ...) Discontinued intervention (give reasons) (n = ...) Analysis Analyzed (n = ...) Excluded from analysis (give reasons) (n = ...) Analyzed (n = ...) Excluded from analysis (give reasons) (n = ...) Academia and Clinic CONSORT 2010 Statement 728 1 June 2010 Annals of Internal Medicine Volume 152 • Number 11 www.annals.org