正在加载图片...
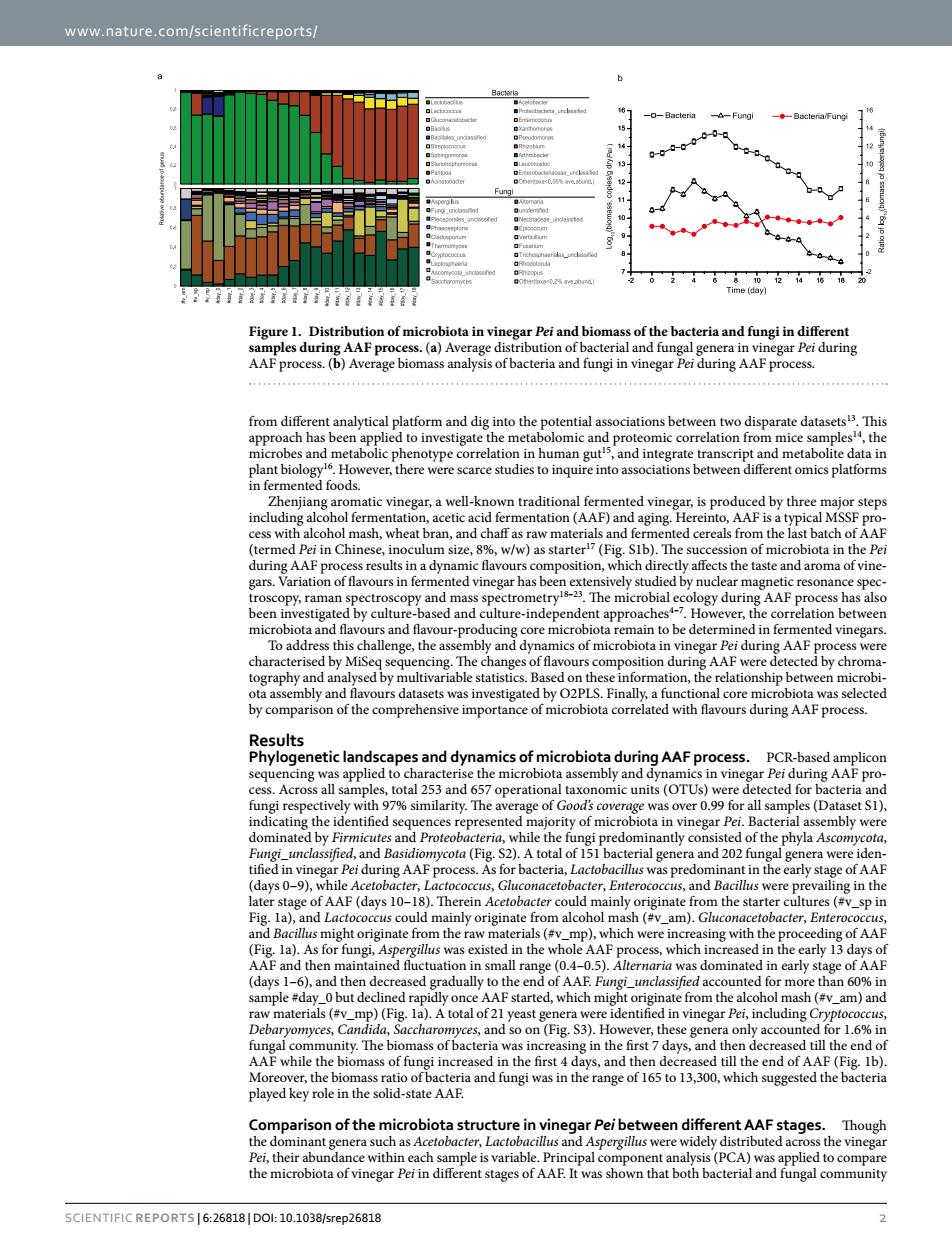
www.nature.com/scientificreports/ Figure 1.Distribu of microbiota in vi gar Pei and bioma s of the bacteria and fungiin different AAF PrO analysis of bacteria and fungi in vine natic vinegar,a well-k mn traditional fermented vine roduced by threemajor steps ran. nd chaff as raw mat nd nted cereals from A position.w ts the tastend ectrom -78 and flavour-proc ain to be detern nted vinegar nge,th were d A cted by chro igated b O2PLS.Finally a functi nal cor ated with flavours during A AF process Result netic landscapes and dynamics of microbiota during AAF process. PCR-hased a ity.The age of G cota (fi 2).A total ofpredom and 202f ly st AA a))Therein a).A total of 21 (#v_am)an onFigs3ntcS r1.6 n the first the hio whichnd of played key role in the solid-state AAF Comparison of the in vinegar Peibetween different AAF stages.Thouh Pei,their SCIENTIEIC REPORTS16-268181D0110 1038/4026818 www.nature.com/scientificreports/ Scientific Reports | 6:26818 | DOI: 10.1038/srep26818 2 from different analytical platform and dig into the potential associations between two disparate datasets13. This approach has been applied to investigate the metabolomic and proteomic correlation from mice samples14, the microbes and metabolic phenotype correlation in human gut15, and integrate transcript and metabolite data in plant biology16. However, there were scarce studies to inquire into associations between different omics platforms in fermented foods. Zhenjiang aromatic vinegar, a well-known traditional fermented vinegar, is produced by three major steps including alcohol fermentation, acetic acid fermentation (AAF) and aging. Hereinto, AAF is a typical MSSF process with alcohol mash, wheat bran, and chaff as raw materials and fermented cereals from the last batch of AAF (termed Pei in Chinese, inoculum size, 8%, w/w) as starter17 (Fig. S1b). The succession of microbiota in the Pei during AAF process results in a dynamic flavours composition, which directly affects the taste and aroma of vinegars. Variation of flavours in fermented vinegar has been extensively studied by nuclear magnetic resonance spectroscopy, raman spectroscopy and mass spectrometry18–23. The microbial ecology during AAF process has also been investigated by culture-based and culture-independent approaches4–7. However, the correlation between microbiota and flavours and flavour-producing core microbiota remain to be determined in fermented vinegars. To address this challenge, the assembly and dynamics of microbiota in vinegar Pei during AAF process were characterised by MiSeq sequencing. The changes of flavours composition during AAF were detected by chromatography and analysed by multivariable statistics. Based on these information, the relationship between microbiota assembly and flavours datasets was investigated by O2PLS. Finally, a functional core microbiota was selected by comparison of the comprehensive importance of microbiota correlated with flavours during AAF process. Results Phylogenetic landscapes and dynamics of microbiota during AAF process. PCR-based amplicon sequencing was applied to characterise the microbiota assembly and dynamics in vinegar Pei during AAF process. Across all samples, total 253 and 657 operational taxonomic units (OTUs) were detected for bacteria and fungi respectively with 97% similarity. The average of Good’s coverage was over 0.99 for all samples (Dataset S1), indicating the identified sequences represented majority of microbiota in vinegar Pei. Bacterial assembly were dominated by Firmicutes and Proteobacteria, while the fungi predominantly consisted of the phyla Ascomycota, Fungi_unclassified, and Basidiomycota (Fig. S2). A total of 151 bacterial genera and 202 fungal genera were identified in vinegar Pei during AAF process. As for bacteria, Lactobacillus was predominant in the early stage of AAF (days 0–9), while Acetobacter, Lactococcus, Gluconacetobacter, Enterococcus, and Bacillus were prevailing in the later stage of AAF (days 10–18). Therein Acetobacter could mainly originate from the starter cultures (#v_sp in Fig. 1a), and Lactococcus could mainly originate from alcohol mash (#v_am). Gluconacetobacter, Enterococcus, and Bacillus might originate from the raw materials (#v_mp), which were increasing with the proceeding of AAF (Fig. 1a). As for fungi, Aspergillus was existed in the whole AAF process, which increased in the early 13 days of AAF and then maintained fluctuation in small range (0.4–0.5). Alternaria was dominated in early stage of AAF (days 1–6), and then decreased gradually to the end of AAF. Fungi_unclassified accounted for more than 60% in sample #day_0 but declined rapidly once AAF started, which might originate from the alcohol mash (#v_am) and raw materials (#v_mp) (Fig. 1a). A total of 21 yeast genera were identified in vinegar Pei, including Cryptococcus, Debaryomyces, Candida, Saccharomyces, and so on (Fig. S3). However, these genera only accounted for 1.6% in fungal community. The biomass of bacteria was increasing in the first 7 days, and then decreased till the end of AAF while the biomass of fungi increased in the first 4 days, and then decreased till the end of AAF (Fig. 1b). Moreover, the biomass ratio of bacteria and fungi was in the range of 165 to 13,300, which suggested the bacteria played key role in the solid-state AAF. Comparison of the microbiota structure in vinegar Pei between different AAF stages. Though the dominant genera such as Acetobacter, Lactobacillus and Aspergillus were widely distributed across the vinegar Pei, their abundance within each sample is variable. Principal component analysis (PCA) was applied to compare the microbiota of vinegar Pei in different stages of AAF. It was shown that both bacterial and fungal community Figure 1. Distribution of microbiota in vinegar Pei and biomass of the bacteria and fungi in different samples during AAF process. (a) Average distribution of bacterial and fungal genera in vinegar Pei during AAF process. (b) Average biomass analysis of bacteria and fungi in vinegar Pei during AAF process