正在加载图片...
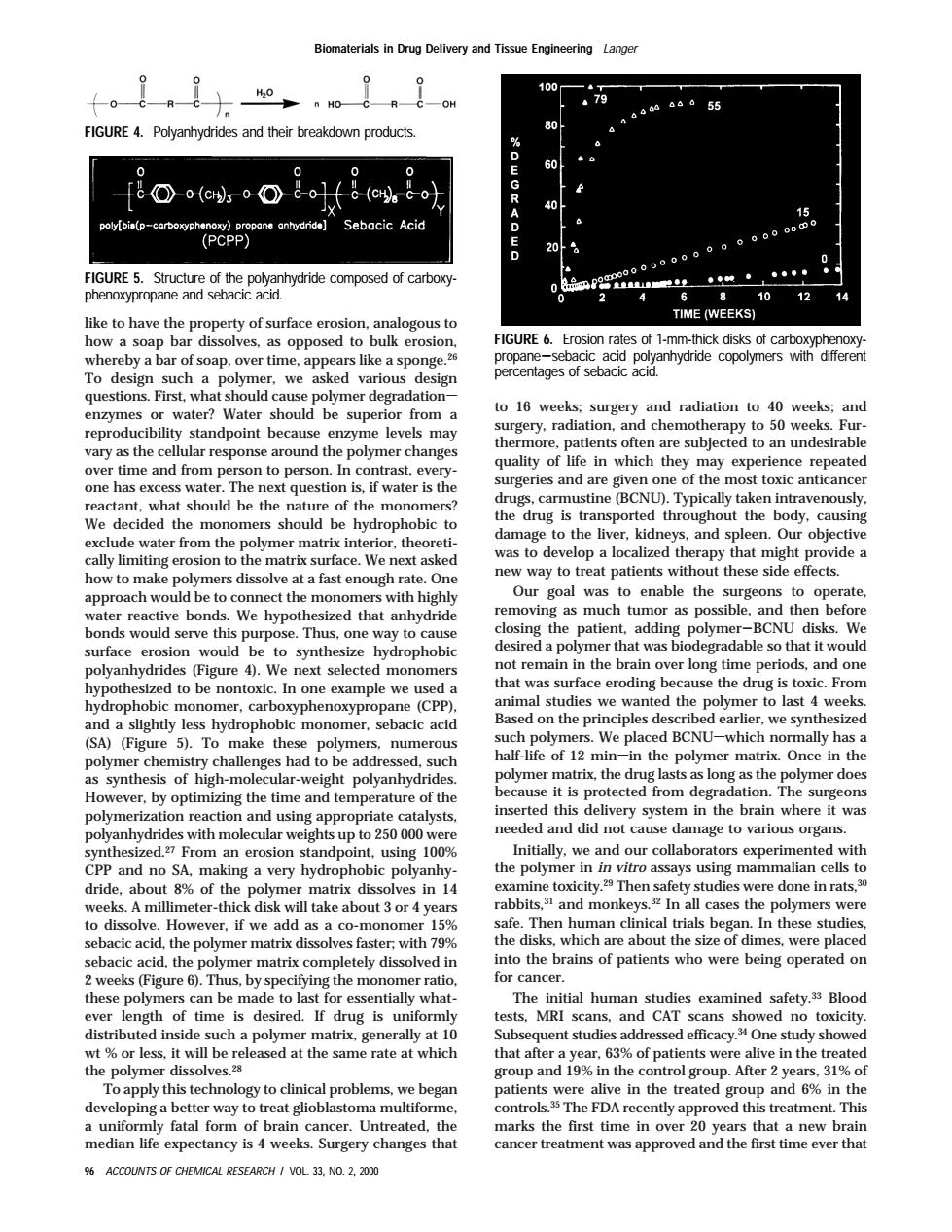
100 OH 80 (PCPP) Sebacic Acid 15 URE 5 12 like to hav e property of surface ero TIME (WEEKS) FIGURE 6.Frosion rates of 1-mm-thick disks of cart by a bar ofs rs lik rent To design such a polymer.we asked various question: irst,wh ld caus to 16 weeks:surger) and radi ation to 40 wee an oducibility standpoint becaus ma vary as the cellular response around the polymer changes oe undesirab quality of life in which they may experience repeated time and from p on【o person I th c antic reactant.what should be the of the ally tal We decided the monomers should be hydrophobic to the drug is transported throughou t the body.caus exclude from the polymer matrix.theoreti damage to the liv Our to top a lo rapy thatm de how to mak polymers dissolve at a fastn ugh rate on ients approachwoldbetoconnecthemonon s with highly the ssible.and then befo rea e bon We hypot that anhydrid closing the patient. adding po ymer-BCNU disks. surface ion would be to synthesize hydrophobic a p gra monomers he ba hypoth that was surface eroding because the drug is toxic.F In one mple we use I studies c polymer last 4 v and a slightly less hydrophobic we sy (SA)(Figure 5 To these numerou alf-life of 12 min which -in the polymer matrix once in the uc ng as the polyme r doe However.by optimizing the time and temperature of the polymerization reactio and using g appropriate catalysts 000 needed and did not cause dan age to various on 100a Initially,we and our collaborators ed with very hydrophobic polvanhy he polyn in vitro assays using mammalian cells 8%of the polyme in 14 31 and 321m wee eter r if we dd 15 Then hum nan clinical trials began.In these studies sebacic acid,the polymer matrix dissolves faster r with 799 dis which are about th sebaci acid,the polym matrix completely dissolved in who s.were place e to last fo ntially what The nitial human studies ever length of time is desired.If drug is uniformly tests .MRI scans,and CAT scans showed no toxicity ributed insid uch polyme uent studi addressed efficacy. One study showe After 2 years.31%of Toapply this technology to clinical problems we began loping a better way to treat glioblasto a multiform FDA median life pectancy is 4 weeks.Surgery changes that cancer treat nt was ovedand the first time ever that ACCOUNTS OF CHEMICAL RESE H/V0L33.N0.2.20 like to have the property of surface erosion, analogous to how a soap bar dissolves, as opposed to bulk erosion, whereby a bar of soap, over time, appears like a sponge.26 To design such a polymer, we asked various design questions. First, what should cause polymer degradations enzymes or water? Water should be superior from a reproducibility standpoint because enzyme levels may vary as the cellular response around the polymer changes over time and from person to person. In contrast, everyone has excess water. The next question is, if water is the reactant, what should be the nature of the monomers? We decided the monomers should be hydrophobic to exclude water from the polymer matrix interior, theoretically limiting erosion to the matrix surface. We next asked how to make polymers dissolve at a fast enough rate. One approach would be to connect the monomers with highly water reactive bonds. We hypothesized that anhydride bonds would serve this purpose. Thus, one way to cause surface erosion would be to synthesize hydrophobic polyanhydrides (Figure 4). We next selected monomers hypothesized to be nontoxic. In one example we used a hydrophobic monomer, carboxyphenoxypropane (CPP), and a slightly less hydrophobic monomer, sebacic acid (SA) (Figure 5). To make these polymers, numerous polymer chemistry challenges had to be addressed, such as synthesis of high-molecular-weight polyanhydrides. However, by optimizing the time and temperature of the polymerization reaction and using appropriate catalysts, polyanhydrides with molecular weights up to 250 000 were synthesized.27 From an erosion standpoint, using 100% CPP and no SA, making a very hydrophobic polyanhydride, about 8% of the polymer matrix dissolves in 14 weeks. A millimeter-thick disk will take about 3 or 4 years to dissolve. However, if we add as a co-monomer 15% sebacic acid, the polymer matrix dissolves faster; with 79% sebacic acid, the polymer matrix completely dissolved in 2 weeks (Figure 6). Thus, by specifying the monomer ratio, these polymers can be made to last for essentially whatever length of time is desired. If drug is uniformly distributed inside such a polymer matrix, generally at 10 wt % or less, it will be released at the same rate at which the polymer dissolves.28 To apply this technology to clinical problems, we began developing a better way to treat glioblastoma multiforme, a uniformly fatal form of brain cancer. Untreated, the median life expectancy is 4 weeks. Surgery changes that to 16 weeks; surgery and radiation to 40 weeks; and surgery, radiation, and chemotherapy to 50 weeks. Furthermore, patients often are subjected to an undesirable quality of life in which they may experience repeated surgeries and are given one of the most toxic anticancer drugs, carmustine (BCNU). Typically taken intravenously, the drug is transported throughout the body, causing damage to the liver, kidneys, and spleen. Our objective was to develop a localized therapy that might provide a new way to treat patients without these side effects. Our goal was to enable the surgeons to operate, removing as much tumor as possible, and then before closing the patient, adding polymer-BCNU disks. We desired a polymer that was biodegradable so that it would not remain in the brain over long time periods, and one that was surface eroding because the drug is toxic. From animal studies we wanted the polymer to last 4 weeks. Based on the principles described earlier, we synthesized such polymers. We placed BCNUswhich normally has a half-life of 12 minsin the polymer matrix. Once in the polymer matrix, the drug lasts as long as the polymer does because it is protected from degradation. The surgeons inserted this delivery system in the brain where it was needed and did not cause damage to various organs. Initially, we and our collaborators experimented with the polymer in in vitro assays using mammalian cells to examine toxicity.29 Then safety studies were done in rats,30 rabbits,31 and monkeys.32 In all cases the polymers were safe. Then human clinical trials began. In these studies, the disks, which are about the size of dimes, were placed into the brains of patients who were being operated on for cancer. The initial human studies examined safety.33 Blood tests, MRI scans, and CAT scans showed no toxicity. Subsequent studies addressed efficacy.34 One study showed that after a year, 63% of patients were alive in the treated group and 19% in the control group. After 2 years, 31% of patients were alive in the treated group and 6% in the controls.35 The FDA recently approved this treatment. This marks the first time in over 20 years that a new brain cancer treatment was approved and the first time ever that FIGURE 4. Polyanhydrides and their breakdown products. FIGURE 5. Structure of the polyanhydride composed of carboxyphenoxypropane and sebacic acid. FIGURE 6. Erosion rates of 1-mm-thick disks of carboxyphenoxypropane-sebacic acid polyanhydride copolymers with different percentages of sebacic acid. Biomaterials in Drug Delivery and Tissue Engineering Langer 96 ACCOUNTS OF CHEMICAL RESEARCH / VOL. 33, NO. 2, 2000