正在加载图片...
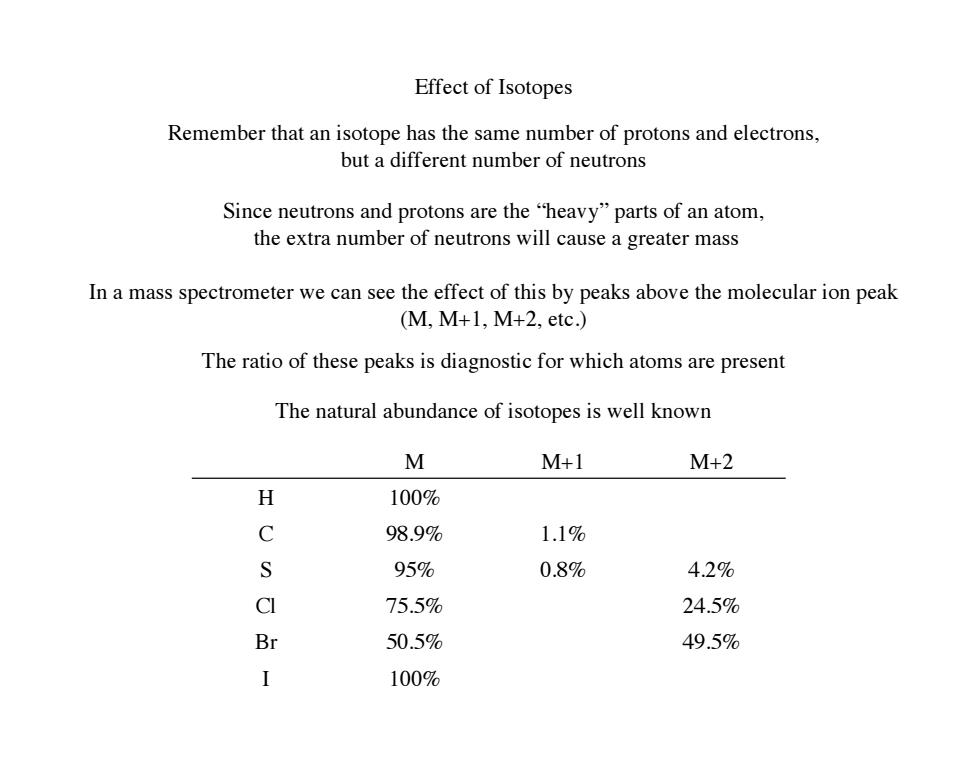
Effect of Isotopes Remember that an isotope has the same number of protons and electrons, but a different number of neutrons Since neutrons and protons are the "heavy"parts of an atom, the extra number of neutrons will cause a greater mass In a mass spectrometer we can see the effect of this by peaks above the molecular ion peak (M,M+1,M+2,etc.) The ratio of these peaks is diagnostic for which atoms are present The natural abundance of isotopes is well known M M+1 M+2 X 100% C 98.9% 1.1% S 95% 0.8% 4.2% CI 75.5% 24.5% Br 50.5% 49.5% I 100%Effect of Isotopes Remember that an isotope has the same number of protons and electrons, but a different number of neutrons Since neutrons and protons are the “heavy” parts of an atom, the extra number of neutrons will cause a greater mass In a mass spectrometer we can see the effect of this by peaks above the molecular ion peak (M, M+1, M+2, etc.) M M+1 M+2 H 100% C 98.9% 1.1% S 95% 0.8% 4.2% Cl 75.5% 24.5% Br 50.5% 49.5% I 100% The ratio of these peaks is diagnostic for which atoms are present The natural abundance of isotopes is well known