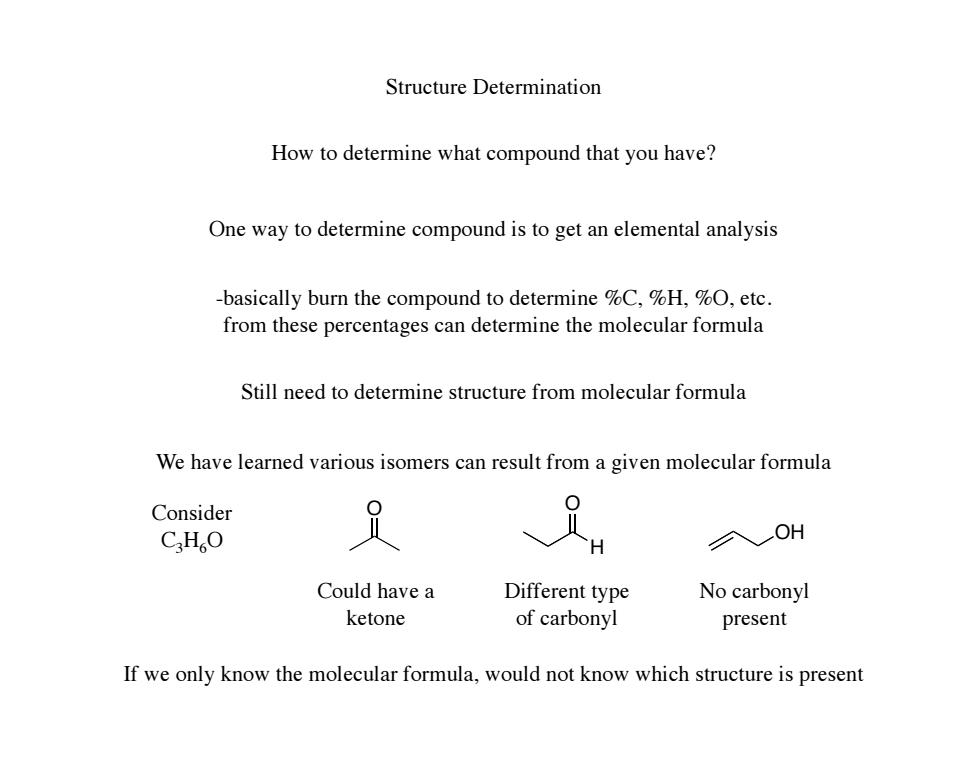
Structure Determination How to determine what compound that you have? One way to determine compound is to get an elemental analysis -basically burn the compound to determine %C,%H,%0,etc. from these percentages can determine the molecular formula Still need to determine structure from molecular formula We have learned various isomers can result from a given molecular formula Consider CHO 、 H OH Could have a Different type No carbonyl ketone of carbonyl present If we only know the molecular formula.would not know which structure is present
Structure Determination How to determine what compound that you have? One way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %O, etc. from these percentages can determine the molecular formula Still need to determine structure from molecular formula We have learned various isomers can result from a given molecular formula Consider C3H6O O O H OH Different type of carbonyl No carbonyl present Could have a ketone If we only know the molecular formula, would not know which structure is present

Structure Determination Even if a pure sample is obtained,how do we know the actual structure of the compound? The development and improvement of analytical instruments to determine structure has been one of the biggest advancements in organic chemistry during the past 60 years Today almost any structure can be determined with these instruments The important part is to recognize what information each instrument provides, and if deciding between possible isomers which technique can be used to differentiate Techniques to be learned: Mass Spectrometry UV-vis Spectroscopy IR Spectroscopy NMR Spectroscopy -mass of compound -conjugation present -functional groups -bond connectivity of -isotopes present structure -distinguish some atoms -symmetry -most important for structure determination
Structure Determination Even if a pure sample is obtained, how do we know the actual structure of the compound? The development and improvement of analytical instruments to determine structure has been one of the biggest advancements in organic chemistry during the past 60 years Today almost any structure can be determined with these instruments The important part is to recognize what information each instrument provides, and if deciding between possible isomers which technique can be used to differentiate Techniques to be learned: Mass Spectrometry UV-vis Spectroscopy IR Spectroscopy NMR Spectroscopy -mass of compound -isotopes present -distinguish some atoms -conjugation present -functional groups -bond connectivity of structure -symmetry -most important for structure determination
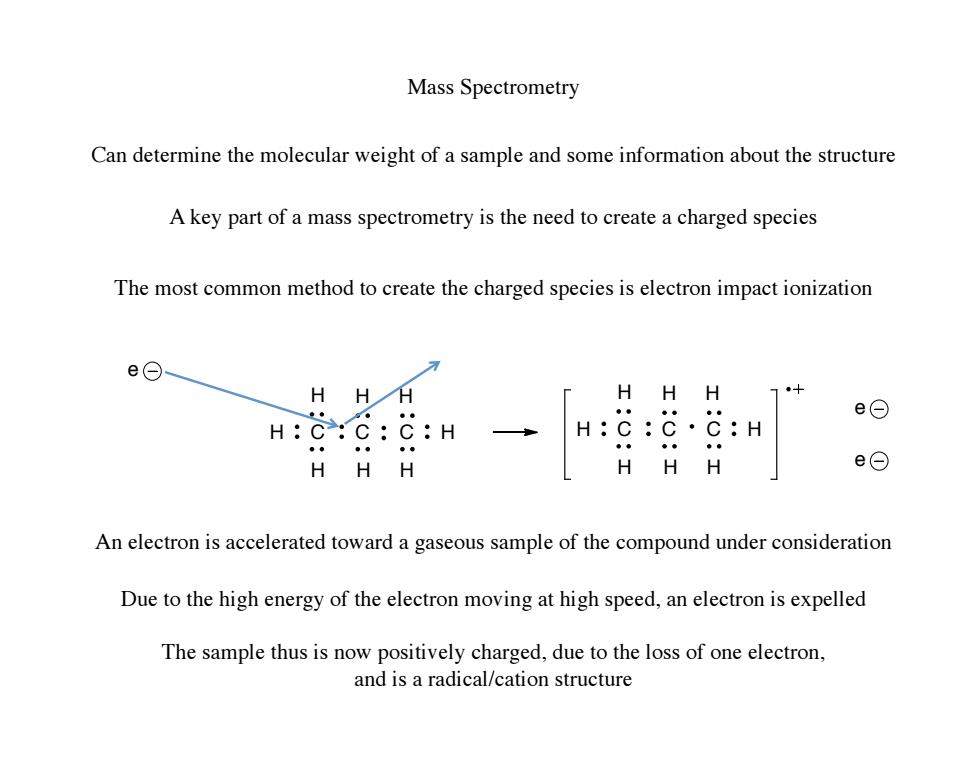
Mass Spectrometry Can determine the molecular weight of a sample and some information about the structure A key part of a mass spectrometry is the need to create a charged species The most common method to create the charged species is electron impact ionization e⊙ H HH H H H 业● e⊙ H:C:C:C:H H:C:C·C:H 。。 HHH HH H e⊙ An electron is accelerated toward a gaseous sample of the compound under consideration Due to the high energy of the electron moving at high speed,an electron is expelled The sample thus is now positively charged,due to the loss of one electron, and is a radical/cation structure
Mass Spectrometry Can determine the molecular weight of a sample and some information about the structure A key part of a mass spectrometry is the need to create a charged species The most common method to create the charged species is electron impact ionization C C C H H H H H H H H C C C H H H H H H H H e e e An electron is accelerated toward a gaseous sample of the compound under consideration Due to the high energy of the electron moving at high speed, an electron is expelled The sample thus is now positively charged, due to the loss of one electron, and is a radical/cation structure
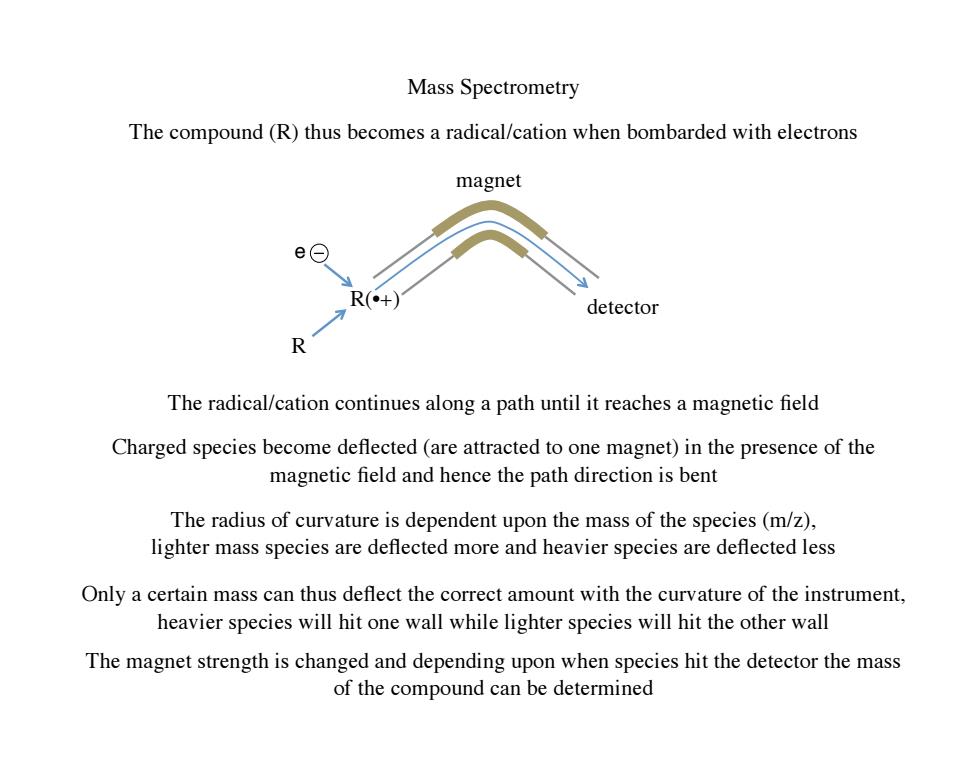
Mass Spectrometry The compound(R)thus becomes a radical/cation when bombarded with electrons magnet e⊙ R(+) detector 个 The radical/cation continues along a path until it reaches a magnetic field Charged species become deflected(are attracted to one magnet)in the presence of the magnetic field and hence the path direction is bent The radius of curvature is dependent upon the mass of the species(m/z), lighter mass species are deflected more and heavier species are deflected less Only a certain mass can thus deflect the correct amount with the curvature of the instrument, heavier species will hit one wall while lighter species will hit the other wall The magnet strength is changed and depending upon when species hit the detector the mass of the compound can be determined
Mass Spectrometry R R(•+) magnet The compound (R) thus becomes a radical/cation when bombarded with electrons The radical/cation continues along a path until it reaches a magnetic field Charged species become deflected (are attracted to one magnet) in the presence of the magnetic field and hence the path direction is bent The radius of curvature is dependent upon the mass of the species (m/z), lighter mass species are deflected more and heavier species are deflected less Only a certain mass can thus deflect the correct amount with the curvature of the instrument, heavier species will hit one wall while lighter species will hit the other wall The magnet strength is changed and depending upon when species hit the detector the mass of the compound can be determined e detector
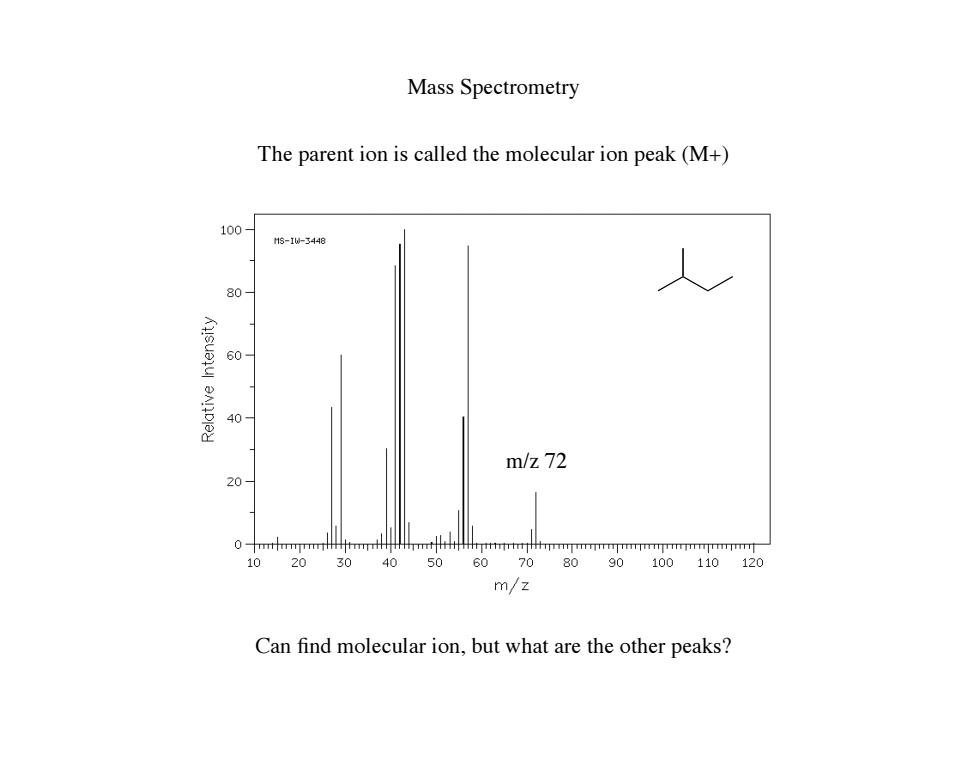
Mass Spectrometry The parent ion is called the molecular ion peak(M+) 100- Hs-1-3448 80 60 40 m/z72 20 0 10 203040 5060708090100110120 m/z Can find molecular ion,but what are the other peaks?
Mass Spectrometry The parent ion is called the molecular ion peak (M+) m/z 72 Can find molecular ion, but what are the other peaks?

Mass Spectrometry The molecular ion peak can fragment Due to the high energy of the radical/cation generated,this species can fragment CH3CH2· m/z72 m/z43 CH3· m/z57 Remember only the charged species will be detected (the radical species will not be affected by the magnetic field) The probability of obtaining a given fragment is due to the STABILITY of the cations produced
The molecular ion peak can fragment Due to the high energy of the radical/cation generated, this species can fragment Remember only the charged species will be detected (the radical species will not be affected by the magnetic field) The probability of obtaining a given fragment is due to the STABILITY of the cations produced Mass Spectrometry m/z 72 CH3CH2 m/z 43 CH3 m/z 57
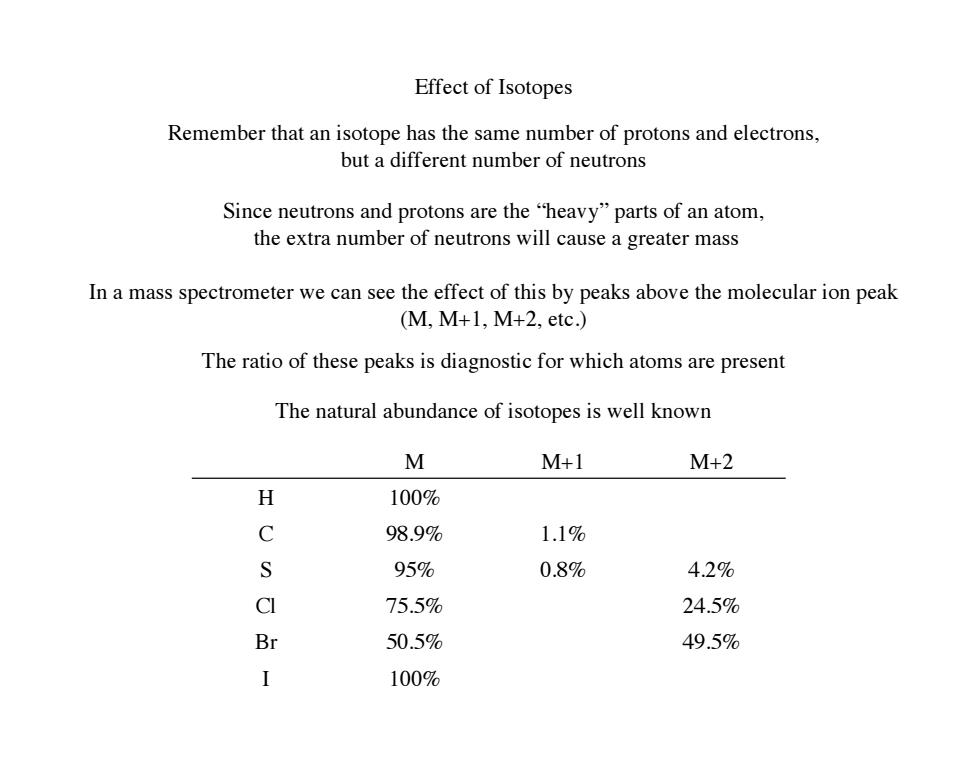
Effect of Isotopes Remember that an isotope has the same number of protons and electrons, but a different number of neutrons Since neutrons and protons are the "heavy"parts of an atom, the extra number of neutrons will cause a greater mass In a mass spectrometer we can see the effect of this by peaks above the molecular ion peak (M,M+1,M+2,etc.) The ratio of these peaks is diagnostic for which atoms are present The natural abundance of isotopes is well known M M+1 M+2 X 100% C 98.9% 1.1% S 95% 0.8% 4.2% CI 75.5% 24.5% Br 50.5% 49.5% I 100%
Effect of Isotopes Remember that an isotope has the same number of protons and electrons, but a different number of neutrons Since neutrons and protons are the “heavy” parts of an atom, the extra number of neutrons will cause a greater mass In a mass spectrometer we can see the effect of this by peaks above the molecular ion peak (M, M+1, M+2, etc.) M M+1 M+2 H 100% C 98.9% 1.1% S 95% 0.8% 4.2% Cl 75.5% 24.5% Br 50.5% 49.5% I 100% The ratio of these peaks is diagnostic for which atoms are present The natural abundance of isotopes is well known

Effect of Isotopes Can distinguish atoms by the ratio of peaks above the molecular ion Especially useful to distinguish which halogen is present Br 人 m/z78 m/z122 m/z170 M/M+2=3 M/M+2=1 100 h3--4951 80 60 40 M/M+2卡1 20 0-tmmpmttrprtimm mmmmjmmphmttimmmmmmmpmmirtpmmmmr 10 20 30 40 50 60708090100110120 m/z
Can distinguish atoms by the ratio of peaks above the molecular ion Especially useful to distinguish which halogen is present Cl Br I m/z 78 M/M+2 = 3 ~ 3/1 m/z 122 M/M+2 = 1 M/M+2 = 1 m/z 170 Effect of Isotopes
Mass Spectrometry Nitrogen Nitrogen is also diagnostic in a mass spectrum due to the odd/even parity of the mass Consider small molecules and their corresponding mass CH m/z=16 NH m/z=17 The molecular ion peak for a molecule with one nitrogen is always odd, all other common atoms in an organic compound yield an even mass
Nitrogen Nitrogen is also diagnostic in a mass spectrum due to the odd/even parity of the mass Consider small molecules and their corresponding mass CH4 m/z = 16 NH3 m/z = 17 The molecular ion peak for a molecule with one nitrogen is always odd, all other common atoms in an organic compound yield an even mass Mass Spectrometry
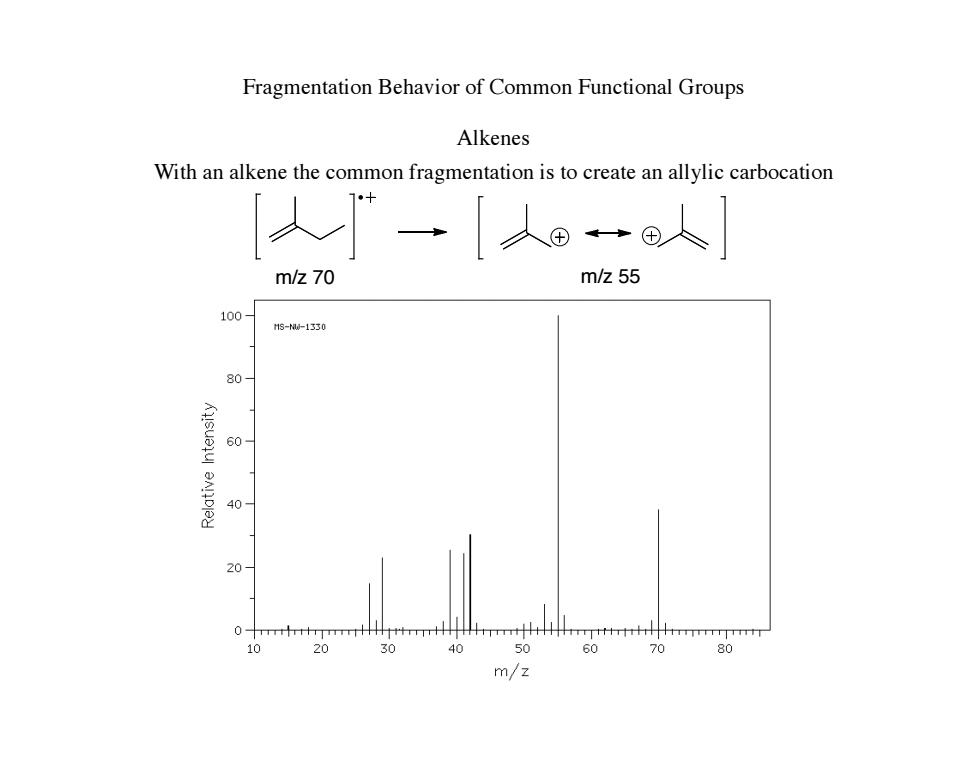
Fragmentation Behavior of Common Functional Groups Alkenes With an alkene the common fragmentation is to create an allylic carbocation 人 ”→人®人 m/z70 m/z55 100 13--1330 80 60 40 10 30 60 70 80 m/z
Fragmentation Behavior of Common Functional Groups Alkenes With an alkene the common fragmentation is to create an allylic carbocation m/z 70 m/z 55