
河北医科大学药学院 三十世纪 天然产物化学的伟大成 ~第一部分~ 李力更教授编辑整理 河北医科大学药学院 天然药物化学教研室 In 20th Century The Great Achievements in Chemistry of Natural Products Part I Department of Medicinal Natural Products College of Phar tical Science Hebei Medical University 天然药物化学教研室 1
河北医科大学药学院 天然药物化学教研室 1 1 二十世纪 天然产物化学的伟大成就 ~第一部分~ 河北医科大学药学院 天然药物化学教研室 李力更 教授 编辑整理 2 In 20th Century The Great Achievements in Chemistry of Natural Products Part I Department of Medicinal Natural Products College of Pharmaceutical Science Hebei Medical University

河北医科大学药学院 Who is he His achievements in organic synthesis were: Cholesterol(胆固醇),Cortisone(可的 松,皮质酮),Lanosterol(羊毛甾醇), Strychnine(士的宁),Lysergie acid(麦角 酸),Reserpine(利血平),Chlorophyll(叶 绿素),Tetracycline(四环素),Colchicine (秋水仙碱),Cephalosporin C(头孢子菌素 C)and Vitamin B2(维生素B12). Who is he And his breathtaking catalog in structure determination includes: Penicillin(青霉素),Strychnine(士的 宁),Patulin(棒曲霉素),Terramycin(土 霉素),Aureomycin(金霉素),Cevine(沙 巴达碱),Magnamycin(碳霉素),Gliotoxin (胶霉毒素),Oleandomycin(竹桃霉素), Streptonigrin(链黑菌素),Tetrodotoxin(河 心豚毒素)· 天然药物化学教研室 2
河北医科大学药学院 天然药物化学教研室 2 3 Who is he ? His achievements in organic synthesis were: Cholesterol(胆固醇),Cortisone(可的 松 , 皮 质 酮 ) , Lanosterol ( 羊 毛 甾 醇 ) , Strychnine(士的宁),Lysergic acid(麦角 酸),Reserpine(利血平),Chlorophyll(叶 绿素),Tetracycline(四环素),Colchicine (秋水仙碱),Cephalosporin C(头孢子菌素 C)and Vitamin B12(维生素B12). 4 And his breathtaking catalog in structure determination includes: Penicillin ( 青 霉 素 ) , Strychnine ( 士 的 宁), Patulin (棒曲霉素),Terramycin(土 霉素), Aureomycin (金霉素),Cevine(沙 巴达碱),Magnamycin(碳霉素),Gliotoxin (胶霉毒素),Oleandomycin(竹桃霉素), Streptonigrin(链黑菌素),Tetrodotoxin(河 豚毒素). Who is he ?

河北医科大学药学院 In 20th century The Noble Winner The master of natural products The foremost synthetic organic chemis Robert Burns WOODWARD (April10,1917-Ji8,979) Boston.Massachusetts fath mother had to work hard to support he 天然药物化学教研室 3
河北医科大学药学院 天然药物化学教研室 3 In 20th century The Noble Winner The master of natural products The foremost synthetic organic chemist Robert Burns WOODWARD (April 10, 1917- July 8, 1979) 6 Bob Woodward was born in Boston, Massachusetts. His father died when Bob was less than two years old, and his mother had to work hard to support her son. Boston, Massachusetts

河北医科大学药学院 Quincy,Massachusetts Bob's early education was in the Ouincy,Massachusetts public schools.During this period he was allowed to skip three years,thus enabling him to finish grammar and high schools in nine years. At age 14,Woodward bought a copy of Ludwig Gattermann's Practical Methods of Organic Chemistry Published by the MacMillan Company,1909 Prof Ludwig Gattermann (1860-1920) Heidelberg University,Germany Later in his life,Woodward did nothing to discourage a persistent legend that he had performed all the experiments in Gattermann's book. 天然药物化学教研室 4
河北医科大学药学院 天然药物化学教研室 4 7 Bob`s early education was in the Quincy, Massachusetts, public schools. During this period he was allowed to skip three years, thus enabling him to finish grammar and high schools in nine years. Quincy, Massachusetts 8 At age 14, Woodward bought a copy of Ludwig Gattermann's Practical Methods of Organic Chemistry. Prof. Ludwig Gattermann ( 1860-1920 ) Heidelberg University, Germany Later in his life, Woodward did nothing to discourage a persistent legend that he had performed all the experiments in Gattermann's book. Published by the MacMillan Company, 1909

河北医科大学药学院 Massachusetts Institute of Technology MIT) “Mind and Hand" nd his s were uraged.In just four ime he was only 20 years old of age. 6 Harvard University Let Plato be your friend and Aristotle, but more let your friend be truth. Woodward joined the chemistry departmentat Harvard University. where he worked until his passed away in 1979. 天然药物化学教研室 5
河北医科大学药学院 天然药物化学教研室 5 9 Massachusetts Institute of Technology ( MIT ) In 1933 at the age of 16, Woodward enrolled in the MIT to study chemistry, although he also had interests at that time in literature, mathematics, and architecture. His unusual talents were soon apparent to the MIT faculty, and his needs for individual study and intensive effort were met and encouraged. In just four years Woodward obtained both bachelor's and doctoral degrees. At that time he was only 20 years old of age. “Mind and Hand” 10 Harvard University Woodward joined the chemistry department at Harvard University, where he worked until his passed away in 1979. Let Plato be your friend and Aristotle, but more let your friend be truth

河北医科大学药学院 Harvard University d of age.in 195 Woodward's achievements in the synthetic organic chemistry 天然药物化学教研室
河北医科大学药学院 天然药物化学教研室 6 11 Harvard University Woodward became a professor at 33 years old of age, in 1950. 12 Woodward`s achievements in the synthetic organic chemistry
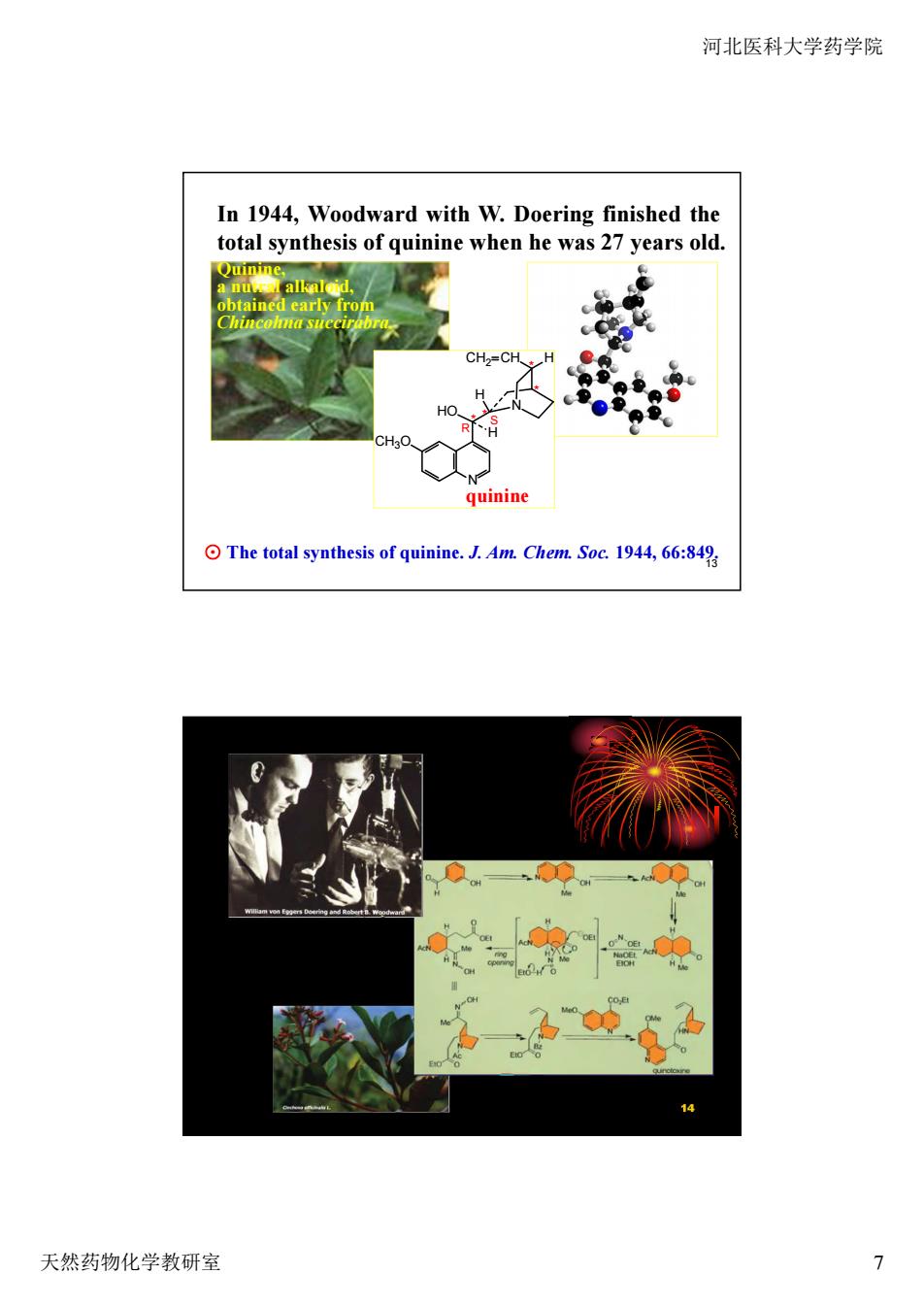
河北医科大学药学院 In 1944,Woodward with W.Doering finished the total synthesis of quinine when he was 27 years old 四 CH.=CH quinine The total synthesis of quinine.J.Am.Chem.Soc.1944,66:849 14 天然药物化学教研室 7
河北医科大学药学院 天然药物化学教研室 7 13 In 1944, Woodward with W. Doering finished the total synthesis of quinine when he was 27 years old. ⊙ The total synthesis of quinine. J. Am. Chem. Soc. 1944, 66:849. Quinine, a nutral alkaloid, obtained early from Chincohna succirabra. * * * * CH3O N HO N CH2 CH H H H quinine R S 14
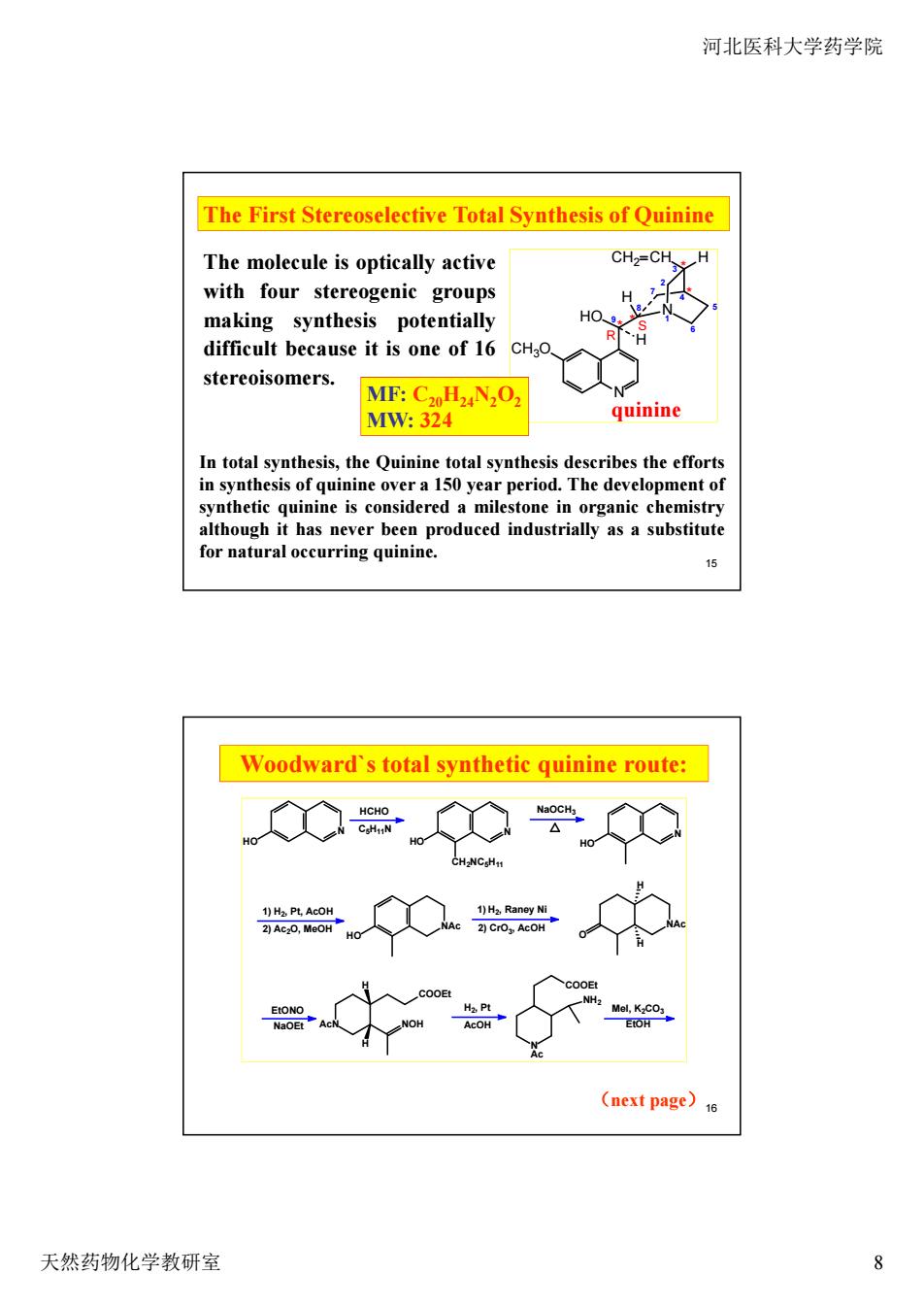
河北医科大学药学院 The First Stereoselective Total Synthesis of Quinine The molecule is optically active CHz=CH. H with four stereogenic groups making synthesis potentially difficult because it is one of 16 CH3o stereoisomers. MF:C2oHzN2Oz MW:324 quinine in fotal synthtsis,the n nine is strially as a substitute or natural occurring quin Woodward's total synthetic quinine route: K0常C学0 器÷0器÷ ÷ (next page)1 天然药物化学教研室 8
河北医科大学药学院 天然药物化学教研室 8 15 * * * * CH3O N HO N CH2 CH H H H quinine R S 1 2 3 4 5 6 7 8 9 The First Stereoselective Total Synthesis of Quinine In total synthesis, the Quinine total synthesis describes the efforts in synthesis of quinine over a 150 year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The molecule is optically active with four stereogenic groups making synthesis potentially difficult because it is one of 16 stereoisomers. MF: C20H24N2O2 MW: 324 16 N HO HCHO C5H11N N HO CH2NC5H11 NaOCH3 N HO 1) H2, Pt, AcOH 2) Ac2O, MeOH HO NAc 1) H2, Raney Ni 2) CrO3, AcOH NAc O H H EtONO NaOEt AcN NOH H H COOEt H2, Pt AcOH N COOEt NH2 Ac MeI, K2CO3 EtOH Woodward`s total synthetic quinine route: (next page)
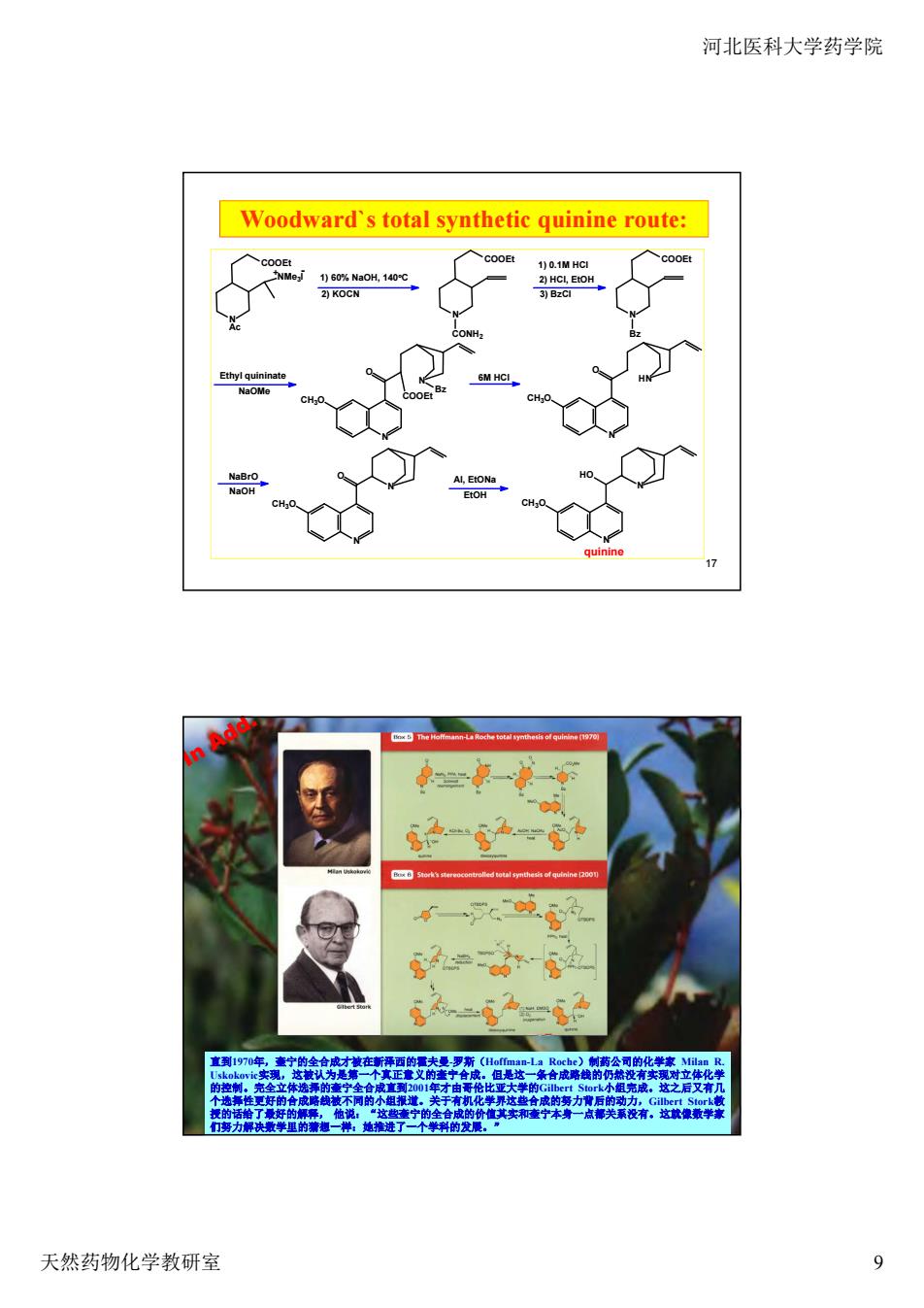
河北医科大学药学院 Woodward's total synthetic quinine route: 总一P一 6一 6学2 =g 遵制,完全 天然药物化学教研室 9
河北医科大学药学院 天然药物化学教研室 9 17 Woodward`s total synthetic quinine route: 1) 60% NaOH, 140o C 2) KOCN N COOEt NMe3I Ac + - N COOEt CONH2 1) 0.1M HCl 2) HCl, EtOH 3) BzCl N COOEt Bz Ethyl quininate NaOMe N CH3O O N COOEt Bz 6M HCl N CH3O O HN NaBrO NaOH N CH3O O N N CH3O HO N quinine Al, EtONa EtOH 18 直到1970年,奎宁的全合成才被在新泽西的霍夫曼-罗斯(Hoffman-La Roche)制药公司的化学家 Milan R. Uskokovic实现,这被认为是第一个真正意义的奎宁合成。但是这一条合成路线的仍然没有实现对立体化学 的控制。完全立体选择的奎宁全合成直到2001年才由哥伦比亚大学的Gilbert Stork小组完成。这之后又有几 个选择性更好的合成路线被不同的小组报道。关于有机化学界这些合成的努力背后的动力,Gilbert Stork教 授的话给了最好的解释, 他说:“这些奎宁的全合成的价值其实和奎宁本身一点都关系没有。这就像数学家 们努力解决数学里的猜想一样:她推进了一个学科的发展
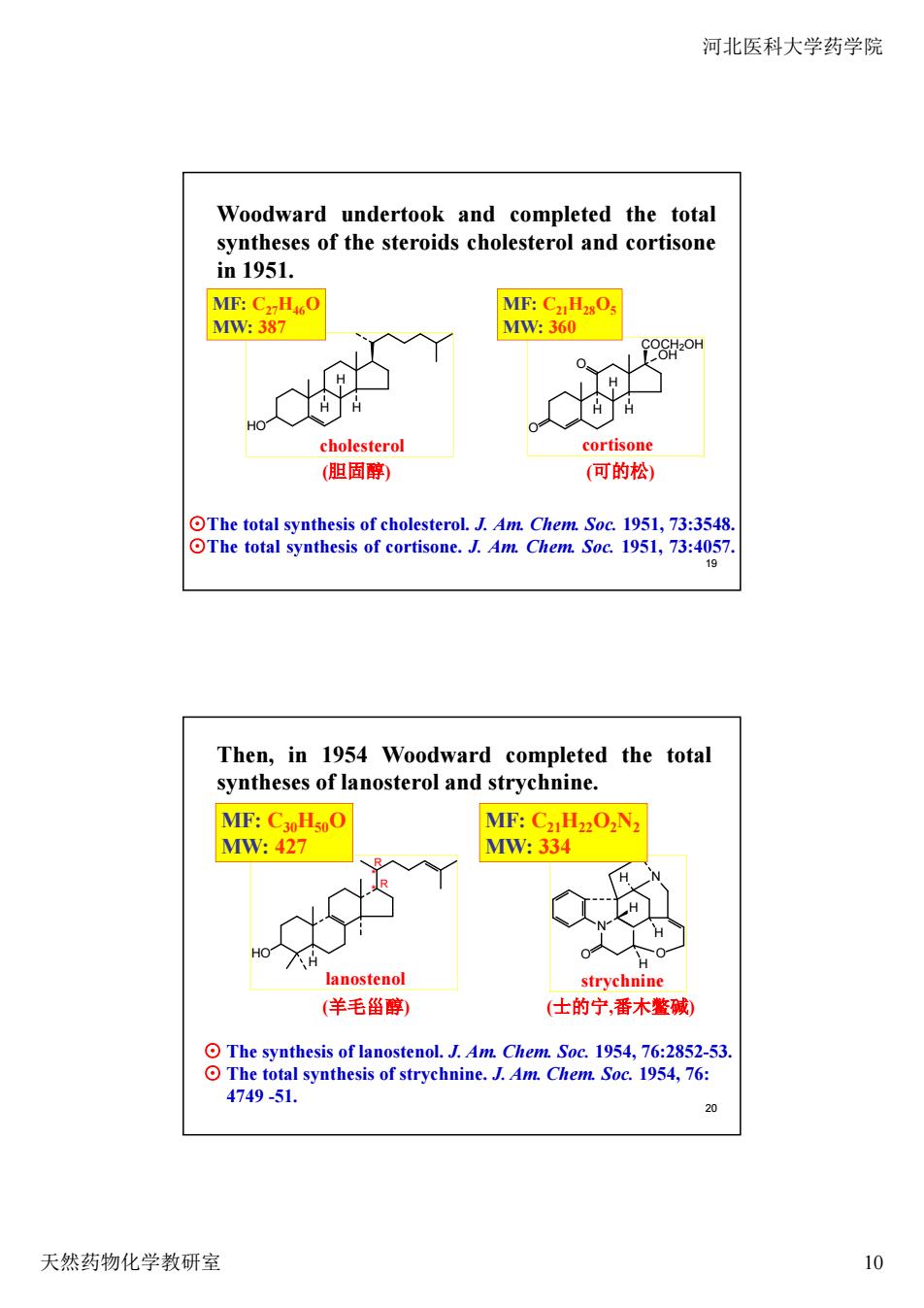
河北医科大学药学院 Woodward undertook and completed the total syntheses of the steroids cholesterol and cortisone in1951. MF:CxHO MF:CaHzOs MW:387 M:360 COCHZOH cholesterol cortisone (胆固酵) (可的松) OThe total synthesis of cholesterol.J.Am.Chem.Soc.1951,73:3548. OThe total synthesis of cortisone.J.Am.Chem.Soc.1951,73:4057. 19 Then,in 1954 Woodward completed the total syntheses of lanosterol and strychnine. MF:CxoHs0O MF:Cz.Hz2O2N2 MM:427 MW:334 H 0 lanostenol strvchnine (羊毛甾醇) 士的宁,番木碱) The synthesis of lanostenolChem Soc.1954.76:2852 al synthesis of strychnine.J.Am.Chem.Soc.1954,76: 4749-51 0 天然药物化学教研室 10
河北医科大学药学院 天然药物化学教研室 10 19 H H H O O OH COCH2OH cholesterol cortisone HO H H H Woodward undertook and completed the total syntheses of the steroids cholesterol and cortisone in 1951. ⊙The total synthesis of cholesterol. J. Am. Chem. Soc. 1951, 73:3548. ⊙The total synthesis of cortisone. J. Am. Chem. Soc. 1951, 73:4057. (胆固醇) (可的松) MF: C27H46O MW: 387 MF: C21H28O5 MW: 360 20 N O O N H H H H strychnine * * HO H R R lanostenol Then, in 1954 Woodward completed the total syntheses of lanosterol and strychnine. ⊙ The synthesis of lanostenol. J. Am. Chem. Soc. 1954, 76:2852-53. ⊙ The total synthesis of strychnine. J. Am. Chem. Soc. 1954, 76: 4749 -51. (羊毛甾醇) (士的宁,番木鳖碱) MF: C30H50O MW: 427 MF: C21H22O2N2 MW: 334